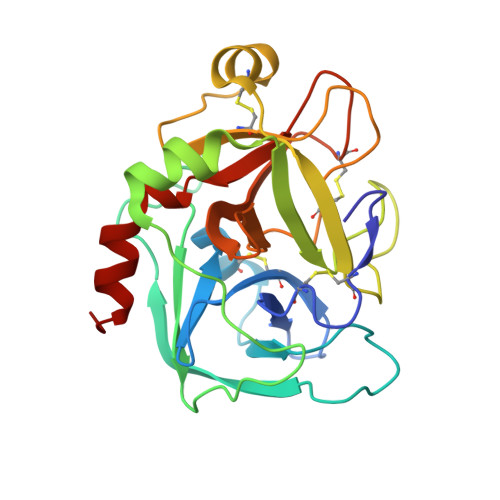
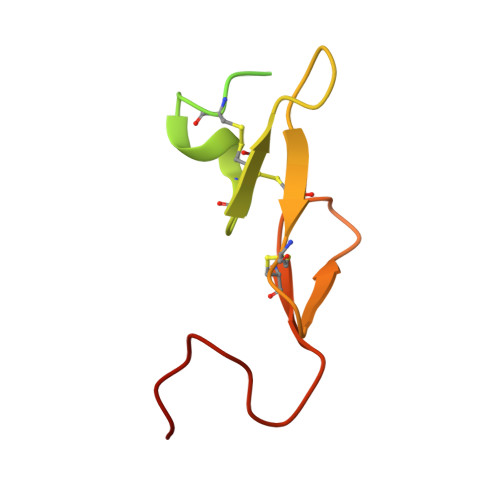
Factor Xa Specific Inhibitor that Induces the Novel Binding Model in Complex with Human Fxa
Matsusue, T., Shiromizu, I., Okamoto, A., Nakayama, K., Nishida, H., Mukaihira, T., Miyazaki, Y., Saitou, F., Morishita, H., Ohnishi, S., Mochizuki, H.To be published.