The crystal structure of the processive endocellulase CelF of Clostridium cellulolyticum in complex with a thiooligosaccharide inhibitor at 2.0 A resolution.
Parsiegla, G., Juy, M., Reverbel-Leroy, C., Tardif, C., Belaich, J.P., Driguez, H., Haser, R.(1998) EMBO J 17: 5551-5562
- PubMed: 9755156
- DOI: https://doi.org/10.1093/emboj/17.19.5551
- Primary Citation of Related Structures:
1FCE - PubMed Abstract:
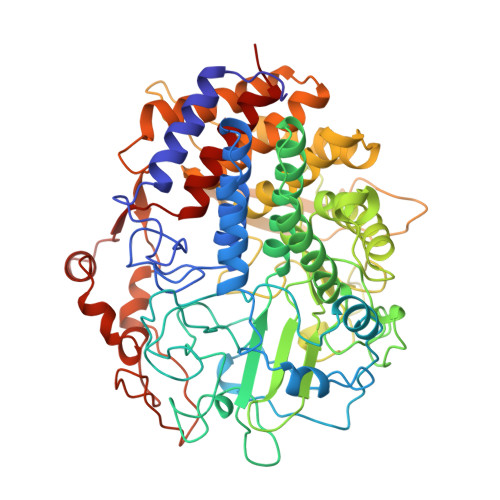
The mesophilic bacterium Clostridium cellulolyticum exports multienzyme complexes called cellulosomes to digest cellulose. One of the three major components of the cellulosome is the processive endocellulase CelF. The crystal structure of the catalytic domain of CelF in complex with two molecules of a thiooligosaccharide inhibitor was determined at 2.0 A resolution. This is the first three-dimensional structure to be solved of a member of the family 48 glycosyl hydrolases. The structure consists of an (alpha alpha)6-helix barrel with long loops on the N-terminal side of the inner helices, which form a tunnel, and an open cleft region covering one side of the barrel. One inhibitor molecule is enclosed in the tunnel, the other exposed in the open cleft. The active centre is located in a depression at the junction of the cleft and tunnel regions. Glu55 is the proposed proton donor in the cleavage reaction, while the corresponding base is proposed to be either Glu44 or Asp230. The orientation of the reducing ends of the inhibitor molecules together with the chain translation through the tunnel in the direction of the active centre indicates that CelF cleaves processively cellobiose from the reducing to the non-reducing end of the cellulose chain.
Organizational Affiliation:
Laboratoire d'Architecture et Fonction des Macromolécules Biologiques, Institut de Biologie Structurale et Microbiologie, Centre National de la Recherche Scientifique, Marseille cedex 20, France.