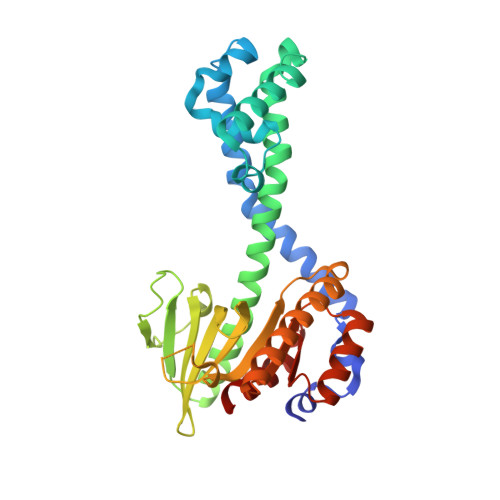
Restriction enzyme BsoBI-DNA complex: a tunnel for recognition of degenerate DNA sequences and potential histidine catalysis.
van der Woerd, M.J., Pelletier, J.J., Xu, S., Friedman, A.M.(2001) Structure 9: 133-144
- PubMed: 11250198
- DOI: https://doi.org/10.1016/s0969-2126(01)00564-0
- Primary Citation of Related Structures:
1DC1 - PubMed Abstract:
Restriction endonucleases form a diverse family of proteins with substantial variation in sequence, structure, and interaction with recognition site DNA. BsoBI is a thermophilic restriction endonuclease that exhibits both base-specific and degenerate recognition within the sequence CPyCGPuG.
Organizational Affiliation:
Department of Biological Sciences, Purdue University, 47907, West Lafayette, IN, USA.