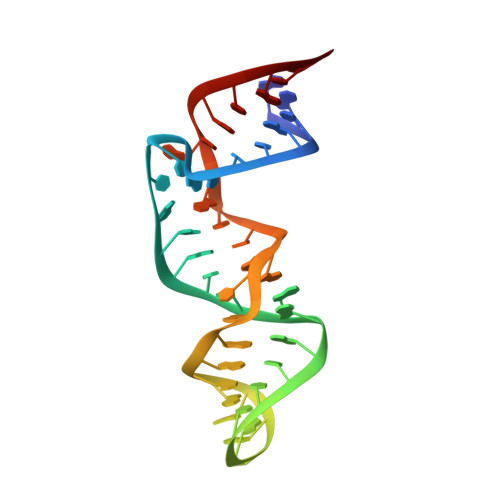
Structure of the phylogenetically most conserved domain of SRP RNA.
Schmitz, U., Behrens, S., Freymann, D.M., Keenan, R.J., Lukavsky, P., Walter, P., James, T.L.(1999) RNA 5: 1419-1429
- PubMed: 10580470
- DOI: https://doi.org/10.1017/s1355838299991458
- Primary Citation of Related Structures:
1CQ5, 1CQL - PubMed Abstract:
The signal recognition particle (SRP) is a phylogenetically conserved ribonucleoprotein required for cotranslational targeting of proteins to the membrane of the endoplasmic reticulum of the bacterial plasma membrane. Domain IV of SRP RNA consists of a short stem-loop structure with two internal loops that contain the most conserved nucleotides of the molecule. All known essential interactions of SRP occur in that moiety containing domain IV. The solution structure of a 43-nt RNA comprising the complete Escherichia coli domain IV was determined by multidimensional NMR and restrained molecular dynamics refinement. Our data confirm the previously determined rigid structure of a smaller subfragment containing the most conserved, symmetric internal loop A (Schmitz et al., Nat Struct Biol, 1999, 6:634-638), where all conserved nucleotides are involved in nucleotide-specific structural interactions. Asymmetric internal loop B provides a hinge in the RNA molecule; it is partially flexible, yet also uniquely structured. The longer strand of internal loop B extends the major groove by creating a ledge-like arrangement; for loop B however, there is no obvious structural role for the conserved nucleotides. The structure of domain IV suggests that loop A is the initial site for the RNA/protein interaction creating specificity, whereas loop B provides a secondary interaction site.
Organizational Affiliation:
Department of Pharmaceutical Chemistry, University of California, San Francisco 94143-0446, USA. schmitz@picasso.ucsf.edu