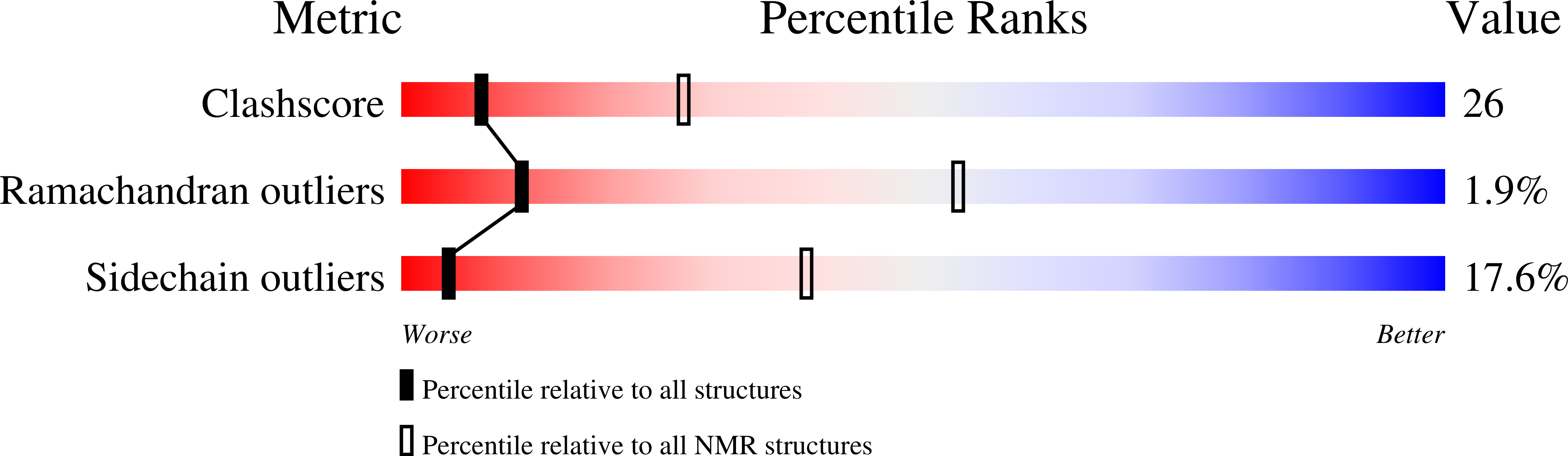Atomic-Resolution Structure of the Protein Encoded by Gene V of fd Bacteriophage in Complex with Viral ssDNA Determined by Magic-Angle Spinning Solid-State NMR.
Shamir, Y., Goldbourt, A.(2023) J Am Chem Soc 145: 300-310
- PubMed: 36542094
- DOI: https://doi.org/10.1021/jacs.2c09957
- Primary Citation of Related Structures:
8ACZ - PubMed Abstract:
F-specific filamentous phages, elongated particles with circular single-stranded DNA encased in a symmetric protein capsid, undergo an intermediate step, where thousands of homodimers of a non-structural protein, gVp, bind to newly synthesized strands of DNA, preventing further DNA replication and preparing the circular genome in an elongated conformation for assembly of a new virion structure at the membrane. While the structure of the free homodimer is known, the ssDNA-bound conformation has yet to be determined. We report an atomic-resolution structure of the gVp monomer bound to ssDNA of fd phage in the nucleoprotein complex elucidated via magic-angle spinning solid-state NMR. The model presents significant conformational changes with respect to the free form. These modifications facilitate the binding mechanism and possibly promote cooperative binding in the assembly of the gVp-ssDNA complex.
Organizational Affiliation:
School of Chemistry, Tel Aviv University, Tel Aviv 6997801, Israel.