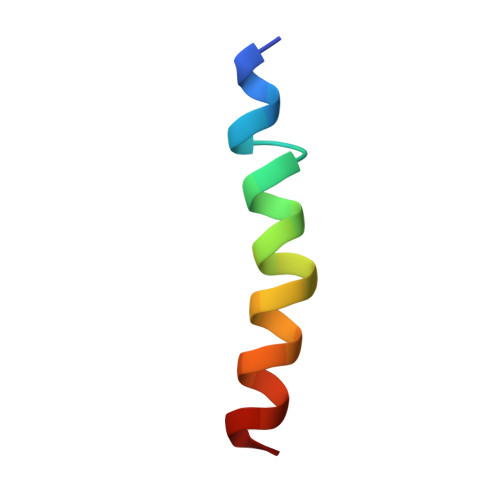
Biophysical study of the structure and dynamics of the antimicrobial peptide maximin 1.
Timmons, P.B., Hewage, C.M.(2022) J Pept Sci 28: e3370-e3370
- PubMed: 34569121
- DOI: https://doi.org/10.1002/psc.3370
- Primary Citation of Related Structures:
7OVZ - PubMed Abstract:
Maximin 1 is a cationic, amphipathic antimicrobial peptide found in the skin secretions and brains of the Chinese red belly toad Bombina maxima. The 27 amino acid residue-long peptide is biologically interesting as it possesses a variety of biological activities, including antibacterial, antifungal, antiviral, antitumour and spermicidal activities. Its three-dimensional structural model was obtained in a 50/50% water/2,2,2-trifluoroethanol-d 3 mixture using two-dimensional NMR spectroscopy. Maximin 1 was found to adopt an α-helical structure from residue Ile 2 to Ala 26 . The peptide is amphipathic, showing a clear separation between polar and non-polar residues. The interactions with sodium dodecyl sulfate micelles, a widely-used bacterial membrane-mimicking environment, were modelled using molecular dynamics simulations. The peptide maintains an α-helical conformation, occasionally displaying a flexibility around the Gly 9 and Gly 16 residues, which is likely responsible for the peptide's low haemolytic activity. It is found to preferentially adopt a position parallel to the micellar surface, establishing a number of hydrophobic and electrostatic interactions with the micelle.
Organizational Affiliation:
UCD School of Biomolecular and Biomedical Science,UCD Centre for Synthesis and Chemical Biology, UCD Conway Institute, University College Dublin, Dublin 4, Ireland.