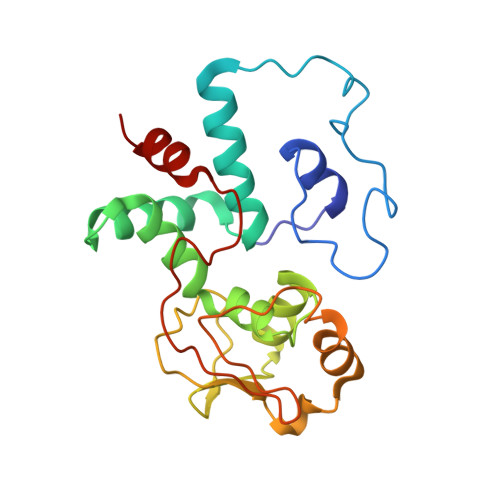
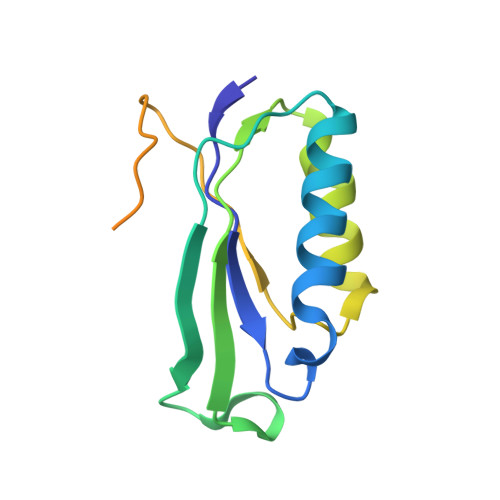
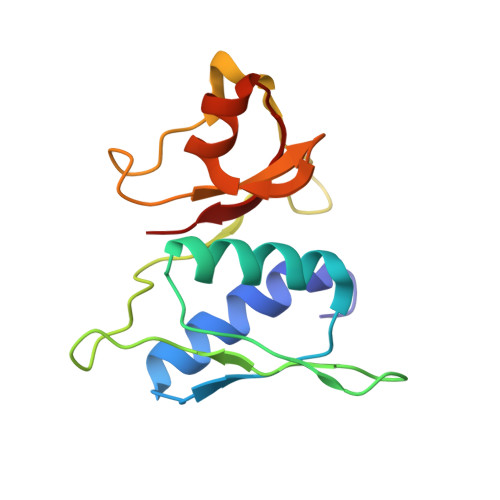
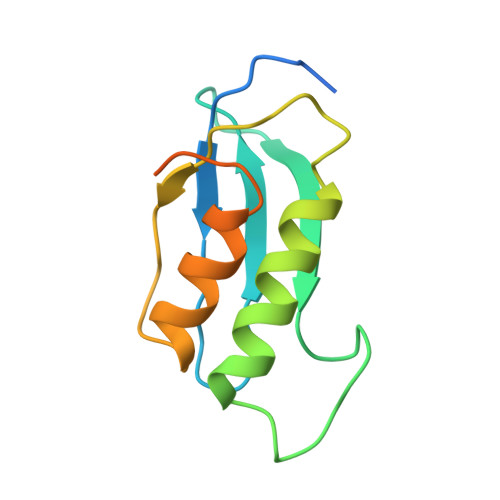
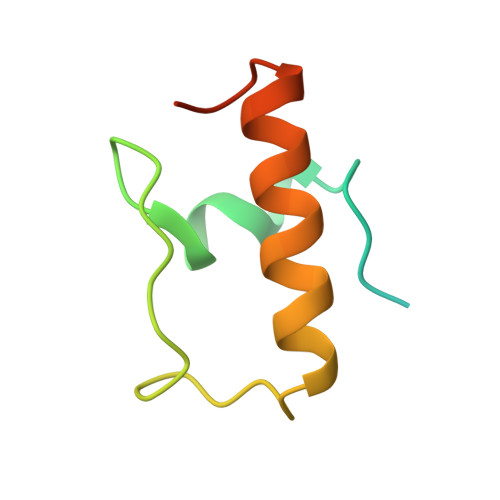
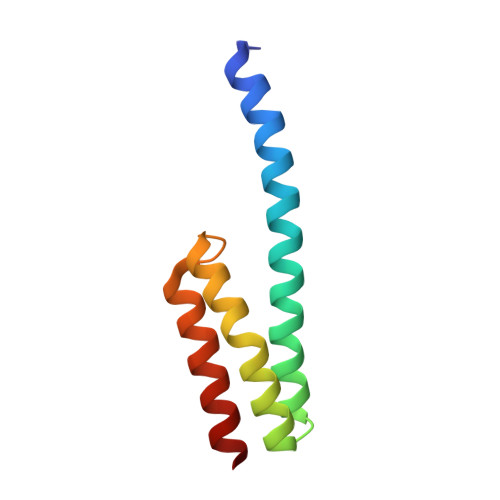
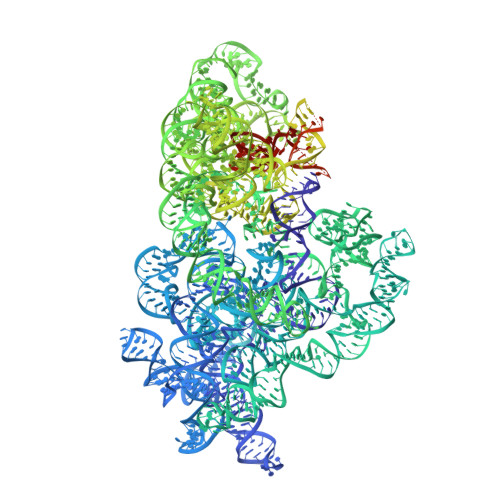
RbfA Is Involved in Two Important Stages of 30S Subunit Assembly: Formation of the Central Pseudoknot and Docking of Helix 44 to the Decoding Center.
Maksimova, E.M., Korepanov, A.P., Kravchenko, O.V., Baymukhametov, T.N., Myasnikov, A.G., Vassilenko, K.S., Afonina, Z.A., Stolboushkina, E.A.(2021) Int J Mol Sci 22
- PubMed: 34200244
- DOI: https://doi.org/10.3390/ijms22116140
- Primary Citation of Related Structures:
7OE0, 7OE1, 7OI0 - PubMed Abstract:
Ribosome biogenesis is a highly coordinated and complex process that requires numerous assembly factors that ensure prompt and flawless maturation of ribosomal subunits. Despite the increasing amount of data collected, the exact role of most assembly factors and mechanistic details of their operation remain unclear, mainly due to the shortage of high-resolution structural information. Here, using cryo-electron microscopy, we characterized 30S ribosomal particles isolated from an Escherichia coli strain with a deleted gene for the RbfA factor. The cryo-EM maps for pre-30S subunits were divided into six classes corresponding to consecutive assembly intermediates: from the particles with a completely unresolved head domain and unfolded central pseudoknot to almost mature 30S subunits with well-resolved body, platform, and head domains and partially distorted helix 44. The structures of two predominant 30S intermediates belonging to most populated classes obtained at 2.7 Å resolutions indicate that RbfA acts at two distinctive 30S assembly stages: early formation of the central pseudoknot including folding of the head, and positioning of helix 44 in the decoding center at a later stage. Additionally, it was shown that the formation of the central pseudoknot may promote stabilization of the head domain, likely through the RbfA-dependent maturation of the neck helix 28. An update to the model of factor-dependent 30S maturation is proposed, suggesting that RfbA is involved in most of the subunit assembly process.
Organizational Affiliation:
Institute of Protein Research, Russian Academy of Sciences, 142290 Pushchino, Russia.