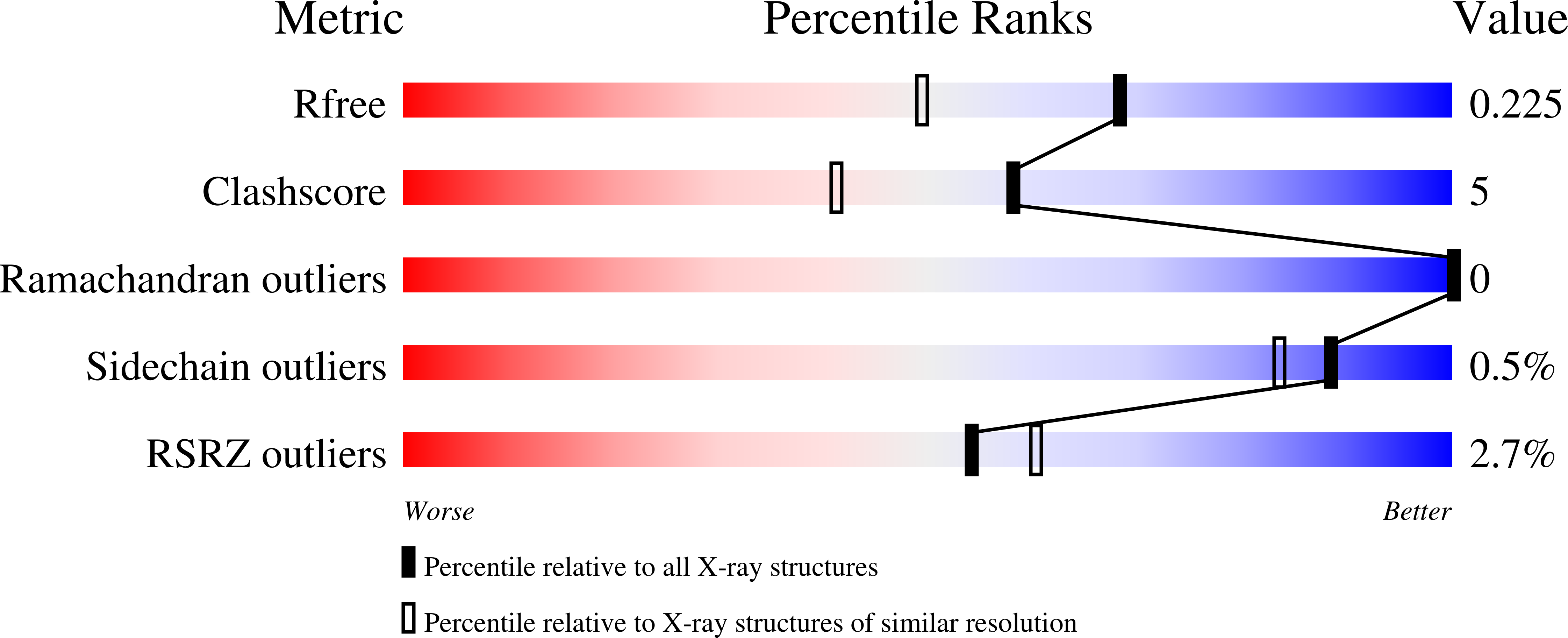
The synthetic varespladib molecule is a multi-functional inhibitor for PLA 2 and PLA 2 -like ophidic toxins.
Salvador, G.H.M., Borges, R.J., Lomonte, B., Lewin, M.R., Fontes, M.R.M.(2021) Biochim Biophys Acta Gen Subj 1865: 129913-129913
- PubMed: 33865953
- DOI: https://doi.org/10.1016/j.bbagen.2021.129913
- Primary Citation of Related Structures:
7LYE - PubMed Abstract:
The treatment for snakebites is early administration of antivenom, which can be highly effective in inhibiting the systemic effects of snake venoms, but is less effective in the treatment of extra-circulatory and local effects. To complement standard-of-care treatments such as antibody-based antivenoms, natural and synthetic small molecules have been proposed for the inhibition of key venom components such as phospholipase A 2 (PLA 2 ) and PLA 2 -like toxins. Varespladib (compound LY315920) is a synthetic molecule developed and clinically tested aiming to block inflammatory cascades of several diseases associated with high PLA 2 s. Recent studies have demonstrated this molecule is able to potently inhibit snake venom catalytic PLA 2 and PLA 2 -like toxins. In vivo and in vitro techniques were used to evaluate the inhibitory effect of varespladib against MjTX-I. X-ray crystallography was used to reveal details of the interaction between these molecules. A new methodology that combines crystallography, mass spectroscopy and phylogenetic data was used to review its primary sequence. Varespladib was able to inhibit the myotoxic and cytotoxic effects of MjTX-I. Structural analysis revealed a particular inhibitory mechanism of MjTX-I when compared to other PLA 2 -like myotoxin, presenting an oligomeric-independent function. Results suggest the effectiveness of varespladib for the inhibition of MjTX-I, in similarity with other PLA 2 and PLA 2 -like toxins. Varespladib appears to be a promissory molecule in the treatment of local effects led by PLA 2 and PLA 2 -like toxins (oligomeric dependent and independent), indicating that this is a multifunctional or broadly specific inhibitor for different toxins within this superfamily.
Organizational Affiliation:
Departamento de Biofísica e Farmacologia, Instituto de Biociências, Universidade Estadual Paulista (UNESP), Botucatu, SP, Brazil.