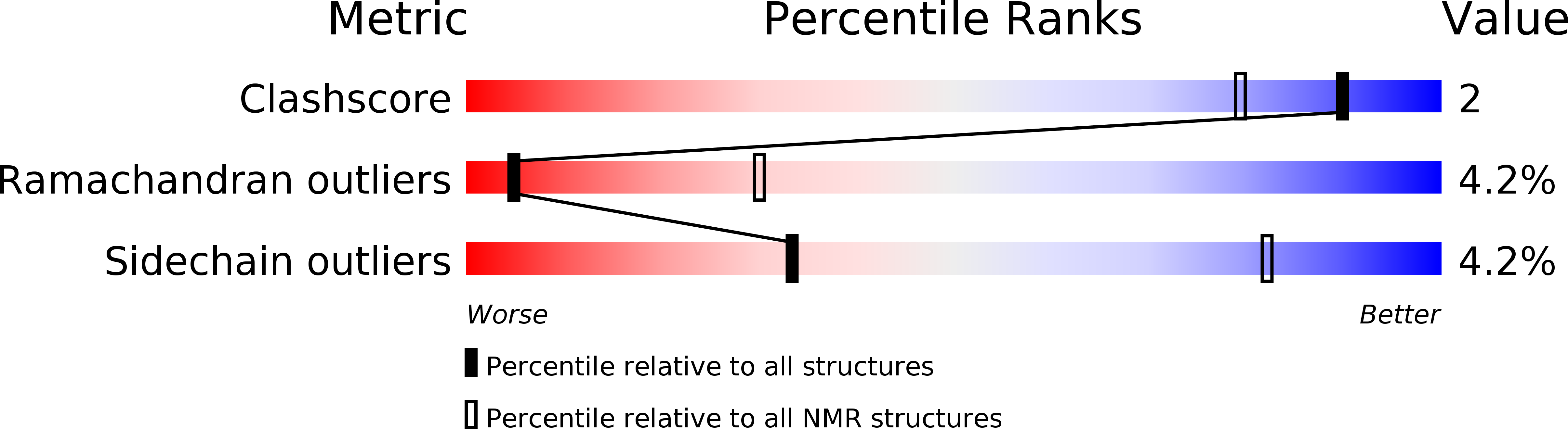PRE-driven protein NMR structures: an alternative approach in highly paramagnetic systems.
Trindade, I.B., Invernici, M., Cantini, F., Louro, R.O., Piccioli, M.(2021) FEBS J 288: 3010-3023
- PubMed: 33124176
- DOI: https://doi.org/10.1111/febs.15615
- Primary Citation of Related Structures:
6XYV, 7A4L, 7A58 - PubMed Abstract:
Metalloproteins play key roles across biology, and knowledge of their structure is essential to understand their physiological role. For those metalloproteins containing paramagnetic states, the enhanced relaxation caused by the unpaired electrons often makes signal detection unfeasible near the metal center, precluding adequate structural characterization right where it is more biochemically relevant. Here, we report a protein structure determination by NMR where two different sets of restraints, one containing Nuclear Overhauser Enhancements (NOEs) and another containing Paramagnetic Relaxation Enhancements (PREs), are used separately and eventually together. The protein PioC from Rhodopseudomonas palustris TIE-1 is a High Potential Iron-Sulfur Protein (HiPIP) where the [4Fe-4S] cluster is paramagnetic in both oxidation states at room temperature providing the source of PREs used as alternative distance restraints. Comparison of the family of structures obtained using NOEs only, PREs only, and the combination of both reveals that the pairwise root-mean-square deviation (RMSD) between them is similar and comparable with the precision within each family. This demonstrates that, under favorable conditions in terms of protein size and paramagnetic effects, PREs can efficiently complement and eventually replace NOEs for the structural characterization of small paramagnetic metalloproteins and de novo-designed metalloproteins by NMR. DATABASES: The 20 conformers with the lowest target function constituting the final family obtained using the full set of NMR restraints were deposited to the Protein Data Bank (PDB ID: 6XYV). The 20 conformers with the lowest target function obtained using NOEs only (PDB ID: 7A58) and PREs only (PDB ID: 7A4L) were also deposited to the Protein Data Bank. The chemical shift assignments were deposited to the BMRB (code 34487).
Organizational Affiliation:
Instituto de Tecnologia Química e Biológica António Xavier (ITQB-NOVA), Universidade Nova de Lisboa, Oeiras, Portugal.