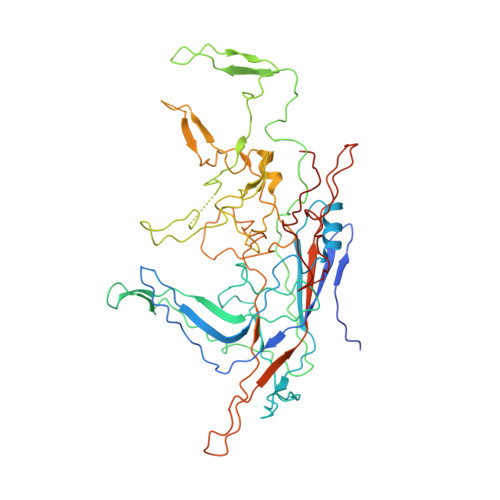
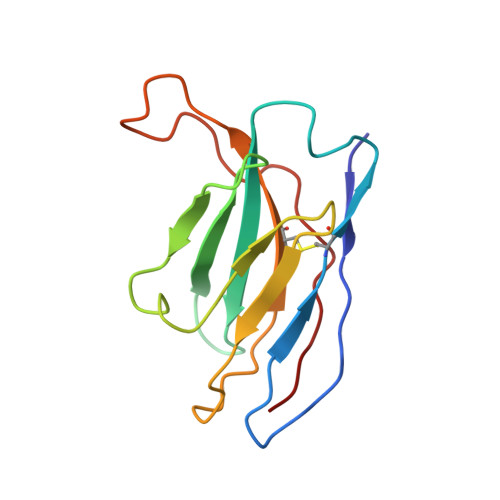
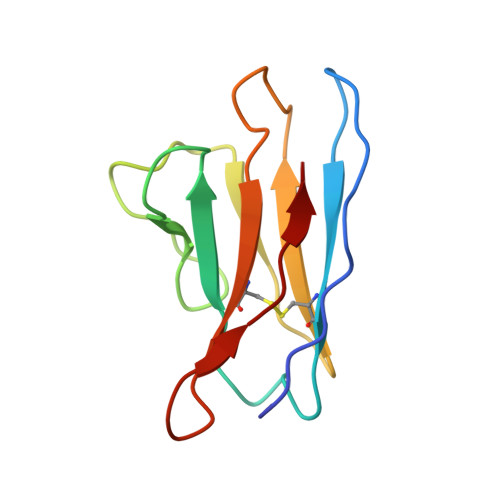
Structure of Parvovirus B19 Decorated by Fabs from a Human Antibody.
Sun, Y., Klose, T., Liu, Y., Modrow, S., Rossmann, M.G.(2019) J Virol 93
- PubMed: 30787153
- DOI: https://doi.org/10.1128/JVI.01732-18
- Primary Citation of Related Structures:
6NN3 - PubMed Abstract:
Parvovirus B19, one of the most common human pathogens, is a small DNA virus that belongs to the Parvoviridae As a result of previous infections, antibodies to B19 are present in most adults. B19 has a strong tropism to erythroid progenitor cells and is able to cause a series of medical conditions, including fifth disease, arthritis, myocarditis, hydrops fetalis, and aplastic crisis. No approved vaccine is currently available for B19, and there is a lack of structural characterization of any B19 epitopes. Here we present the first cryo-electron microscopy (cryo-EM) structure of a B19 virus-like particle (VLP) complexed with the antigen-binding fragment (Fab) of a human neutralizing antibody, 860-55D. A model was built into the 3.2-Å-resolution map, and the antigenic residues on the surface of the B19 capsid were identified. Antibody 860-55D bridges the capsid of B19 by binding to a quaternary structure epitope formed by residues from three neighboring VP2 capsid proteins. IMPORTANCE Parvovirus B19 is a common human pathogen and a particular threat to children, pregnant women, and patients with sickle cell disease or AIDS. Currently, neutralizing antibody is the most efficient treatment for acute B19 infections. Research on the antigenic properties of B19 will guide the usage of these antibodies and facilitate vaccine development. We have determined and report here the high-resolution structure of B19 virus-like particles (VLPs) complexed with the Fab of a human neutralizing antibody. The structure shows a quaternary structure epitope formed by three VP2 proteins and provides details on host recognition of human B19 virus.
Organizational Affiliation:
Department of Biological Sciences, Purdue University, West Lafayette, Indiana, USA.