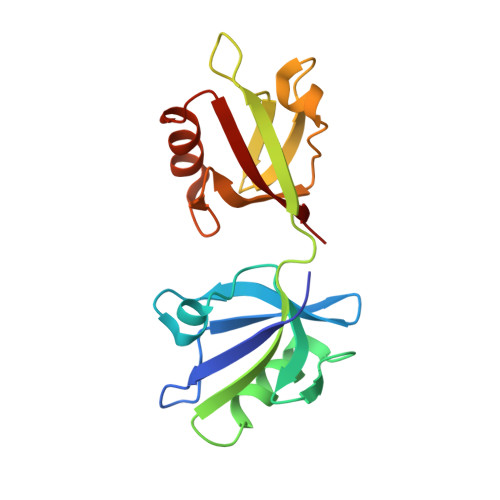
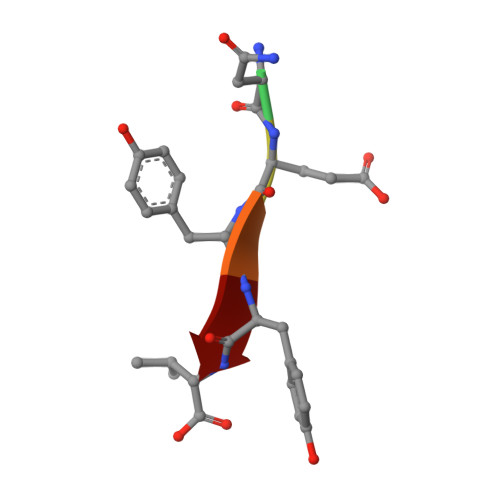
The Binding of the Pdz Tandem of Syntenin to Target Proteins.
Grembecka, J., Cierpicki, T., Devedjiev, Y., Derewenda, U., Kang, B.S., Bushweller, J.H., Derewenda, Z.S.(2006) Biochemistry 45: 3674
- PubMed: 16533050
- DOI: https://doi.org/10.1021/bi052225y
- Primary Citation of Related Structures:
1V1T, 1W9E, 1W9O, 1W9Q, 1YBO - PubMed Abstract:
PDZ domains are among the most abundant protein modules in the known genomes. Their main function is to provide scaffolds for membrane-associated protein complexes by binding to the cytosolic, C-terminal fragments of receptors, channels, and other integral membrane proteins. Here, using both heteronuclear NMR and single crystal X-ray diffraction, we show how peptides with different sequences, including those corresponding to the C-termini of syndecan, neurexin, and ephrin B, can simultaneously bind to both PDZ domains of the scaffolding protein syntenin. The PDZ2 domain binds these peptides in the canonical fashion, and an induced fit mechanism allows for the accommodation of a range of side chains in the P(0) and P(-)(2) positions. However, binding to the PDZ1 domain requires that the target peptide assume a noncanonical conformation. These data help explain how syntenin, and perhaps other PDZ-containing proteins, may preferentially bind to dimeric and clustered targets, and provide a mechanistic explanation for the previously reported cooperative ligand binding by syntenin's two PDZ domains.
Organizational Affiliation:
Department of Molecular Physiology and Biological Physics, University of Virginia School of Medicine, Charlottesville, Virginia 22908-0736, USA.