Deterministic chaos in the self-assembly of beta sheet nanotubes from an amphipathic oligopeptide.
Wang, F., Gnewou, O., Wang, S., Osinski, T., Zuo, X., Egelman, E.H., Conticello, V.P.(2021) Matter 4: 3217-3231
- PubMed: 34632372
- DOI: https://doi.org/10.1016/j.matt.2021.06.037
- Primary Citation of Related Structures:
7LQE, 7LQF, 7LQG, 7LQH, 7LQI - PubMed Abstract:
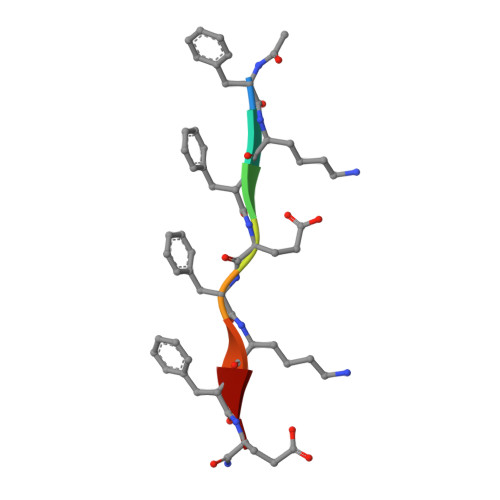
The self-assembly of designed peptides into filaments and other higher-order structures has been the focus of intense interest because of the potential for creating new biomaterials and biomedical devices. These peptide assemblies have also been used as models for understanding biological processes, such as the pathological formation of amyloid. We investigate the assembly of an octapeptide sequence, Ac-FKFEFKFE-NH 2 , motivated by prior studies that demonstrated that this amphipathic β strand peptide self-assembled into fibrils and biocompatible hydrogels. Using high-resolution cryoelectron microscopy (cryo-EM), we are able to determine the atomic structure for two different coexisting forms of the fibrils, containing four and five β sandwich protofilaments, respectively. Surprisingly, the inner walls in both forms are parallel β sheets, while the outer walls are antiparallel β sheets. Our results demonstrate the chaotic nature of peptide self-assembly and illustrate the importance of cryo-EM structural analysis to understand the complex phase behavior of these materials at near-atomic resolution.
Organizational Affiliation:
Department of Biochemistry and Molecular Genetics, University of Virginia, Charlottesville, VA 22908, USA.