Phosphinanes and Azaphosphinanes as Potent and Selective Inhibitors of Activated Thrombin-Activatable Fibrinolysis Inhibitor (TAFIa).
Schaffner, A.P., Sansilvestri-Morel, P., Despaux, N., Ruano, E., Persigand, T., Rupin, A., Mennecier, P., Vallez, M.O., Raimbaud, E., Desos, P., Gloanec, P.(2021) J Med Chem 64: 3897-3910
- PubMed: 33764059
- DOI: https://doi.org/10.1021/acs.jmedchem.0c02072
- Primary Citation of Related Structures:
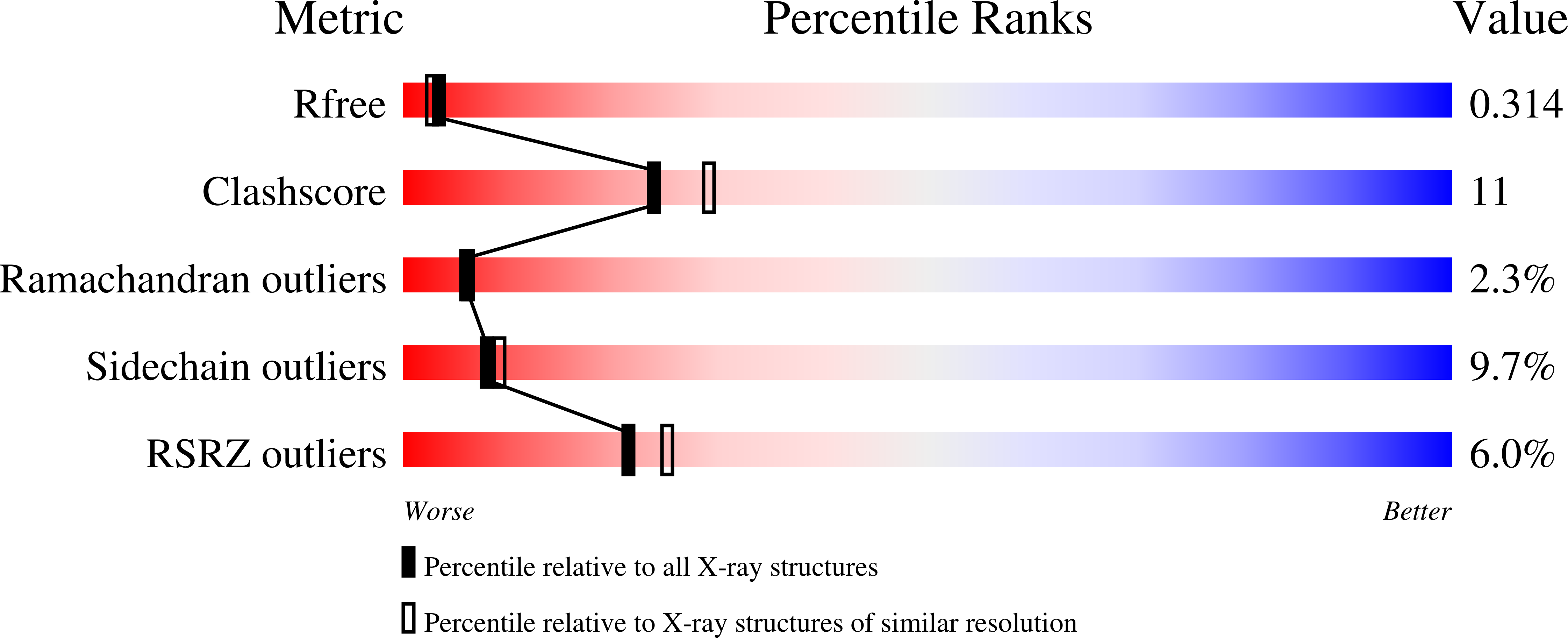
7NEE, 7NEU - PubMed Abstract:
Selective and potent inhibitors of activated thrombin activatable fibrinolysis inhibitor (TAFIa) have the potential to increase endogenous and therapeutic fibrinolysis and to behave like profibrinolytic agents without the risk of major hemorrhage, since they do not interfere either with platelet activation or with coagulation during blood hemostasis. Therefore, TAFIa inhibitors could be used in at-risk patients for the treatment, prevention, and secondary prevention of stroke, venous thrombosis, and pulmonary embolisms. In this paper, we describe the design, the structure-activity relationship (SAR), and the synthesis of novel, potent, and selective phosphinanes and azaphosphinanes as TAFIa inhibitors. Several highly active azaphosphinanes display attractive properties suitable for further in vivo efficacy studies in thrombosis models.
Organizational Affiliation:
Institut de Recherches Servier, 11 rue des Moulineaux, 92150 Suresnes, et 125 Chemin de Ronde, 78290 Croissy-sur-Seine, France.