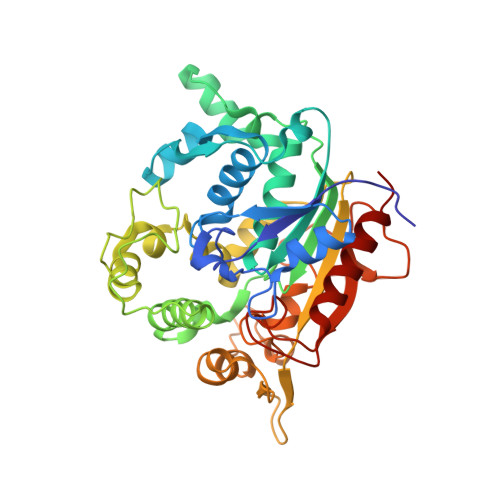
X-ray structure and characterization of a thermostable lipase from Geobacillus thermoleovorans.
Moharana, T.R., Pal, B., Rao, N.M.(2019) Biochem Biophys Res Commun 508: 145-151
- PubMed: 30471860
- DOI: https://doi.org/10.1016/j.bbrc.2018.11.105
- Primary Citation of Related Structures:
6A12 - PubMed Abstract:
Thermo-alkalophilic bacterium, Geobacillus thermoleovorans secrets many enzymes including a 43 kDa extracellular lipase. Significant thermostability, organic solvent stability and wide substrate preferences for hydrolysis drew our attention to solve its structure by crystallography. The structure was solved by molecular replacement method and refined up to 2.14 Å resolution. Structure of the lipase showed an alpha-beta fold with 19 α-helices and 10 β-sheets. The active site remains covered by a lid. One calcium and one zinc atom was found in the crystal. The structure showed a major difference (rmsd 5.6 Å) from its closest homolog in the amino acid region 191 to 203. Thermal unfolding of the lipase showed that the lipase is highly stable with T m of 76 °C. 13 C NMR spectra of products upon triglyceride hydrolysate revealed that the lipase hydrolyses at both sn-1 and sn-2 positions with equal efficiency.
Organizational Affiliation:
CSIR- Centre for Cellular and Molecular Biology, Uppal Road, Hyderabad, 500007, India.