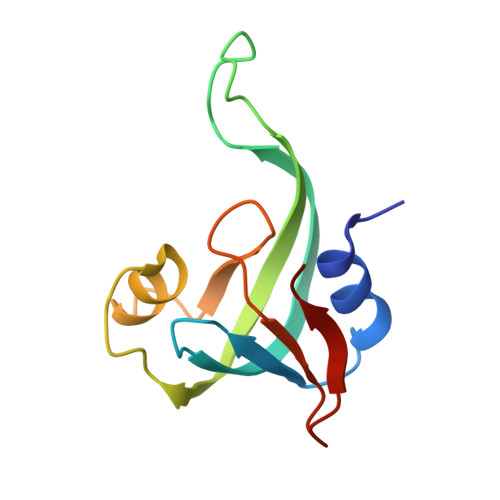
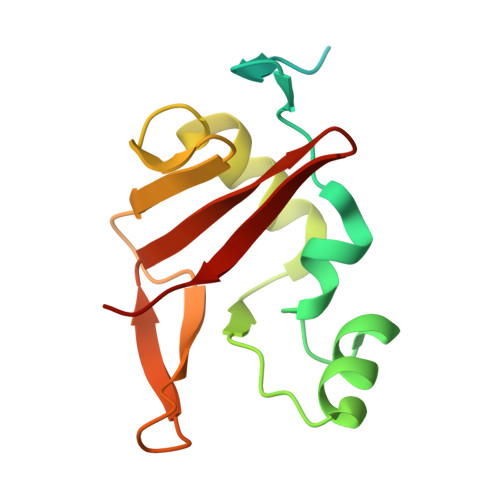
The yxiD gene from Bacillus subtilis 6633 encodes a polymorphic toxin with tRNase activity and is neutralized by the cognate immunity protein YxxD.
Rohilla, R., Kaundal, S., Thakur, K.G.(2025) Nucleic Acids Res 53
- PubMed: 41385326
- DOI: https://doi.org/10.1093/nar/gkaf1321
- Primary Citation of Related Structures:
9V8I - PubMed Abstract:
Polymorphic toxin systems (PTS) are widespread and play an important role in bacterial competition and shaping communities. However, these systems are less studied in Gram-positive bacteria. Here, we structurally and functionally characterized YxiD-YxxD, a predicted member of PTS from Bacillus subtilis 6633. Using growth curve assays, we established that YxiD-YxxD codes for a toxin-immunity protein pair. We determined a 1.7 Å resolution crystal structure of the YxiDCTDH528A (C-terminal domain of YxiD) toxin bound to its cognate immunity protein YxxD. Structure revealed that the toxin adopts a Barnase/EndoU/colicin/RelE (BECR) fold, a characteristic of RNase toxins and the immunity protein sterically occludes binding of the molecular substrate to neutralize the toxin. Structural and other biophysical studies revealed that YxiDCTD-YxxD forms a stable 1:1 stoichiometric complex with KD of ∼9.4 nM. RNA Sequencing experiments revealed that expression of toxin results in downregulation of several tRNAs and essential genes involved in cell wall biosynthesis, resulting in cellular toxicity. We further demonstrate that YxiDCTD is a metal ion-dependent tRNase that cleaves several tRNAs. Taken together, our study provides the structural basis for YxiD neutralization by cognate immunity protein YxxD and establishes YxiDCTD toxin as a metal-dependent tRNase.
- Structural Biology Laboratory, CSIR-Institute of Microbial Technology, Chandigarh 160036, India.
Organizational Affiliation: