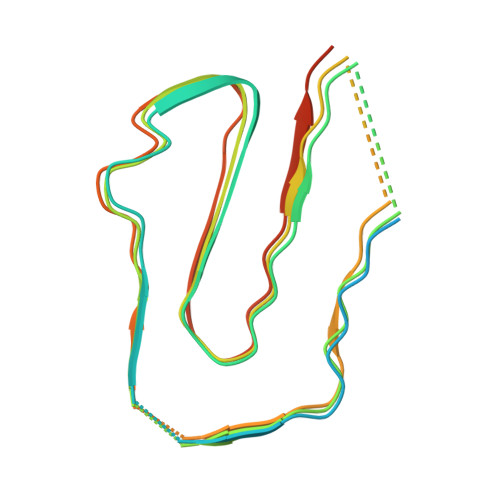
Natural Design of a Stabilized Cross-beta Fold: Structure of the FuA FapC from Pseudomonas Sp. UK4 Reveals a Critical Role for Stacking of Imperfect Repeats.
Jiang, Y., Pena-Diaz, S., Zhang, Z., Daugberg, A.O.H., Lopez Hernandez, M., Nielsen, J., Huang, Q., Qin, S., Dueholm, M.K.D., Dong, M., Pedersen, J.S., Cao, Q., Otzen, D.E., Wang, H.(2025) Adv Mater 37: e2505503-e2505503
- PubMed: 40495649
- DOI: https://doi.org/10.1002/adma.202505503
- Primary Citation of Related Structures:
9U4U - PubMed Abstract:
An essential structural component of bacterial biofilms is functional amyloid (FuA), which also has great potential as an engineerable nano-biomaterial. However, experimentally based high resolution structures of FuA that resolve individual residues are lacking. A fully experimentally based 3.2 Å resolution cryo-electron microscopy density map of the FuA protein FapC from Pseudomonas sp. UK4 is presented, which reveals a Greek key-shaped protofilament. The structure supports bioinformatic identification of conserved motifs and is broadly consistent with the AlphaFold prediction but with important modifications. Each FapC monomer consists of three imperfect repeats (IRs), with each repeat forming one cross-β layer. An array of highly conserved Asn and Gln residues with an extensive H-bonding network underpins this conserved Greek key-shape and reveals the role of heterogeneous cross-β stacking in amyloid cross-seeding. The covariation of residues in the hydrophobic core among different IRs suggests a cooperative monomer folding process during fibril elongation, while heterogeneous stacking of IRs reduces charge repulsion between layers to stabilize the monomer fold. The FapC fibrils show intrinsic catalytic activity and strain-dependent nanomechanical properties. Combined with mutagenesis data, the structure provides mechanistic insights into formation of FapC FuA from disordered monomers and a structural foundation for the design of novel biomaterials.
- Department of Clinical Laboratory, the First Affiliated Hospital of Guangxi Medical University, Key Laboratory of Clinical Laboratory Medicine of Guangxi Department of Education, Guangxi Key Laboratory of Enhanced Recovery after Surgery for Gastrointestinal Cancer, Shuangyong Road 6, Guangxi Zhuang Autonomous Region, Nanning, 530021, China.
Organizational Affiliation: