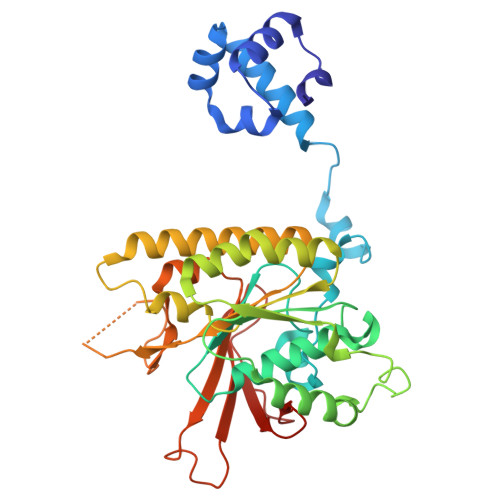
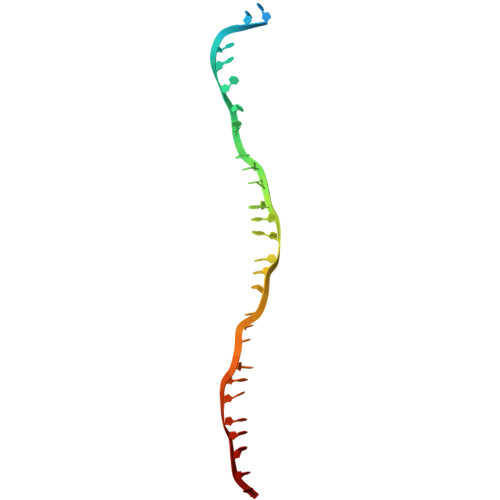
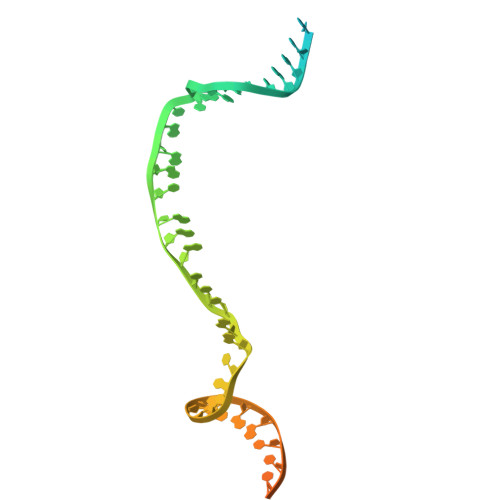
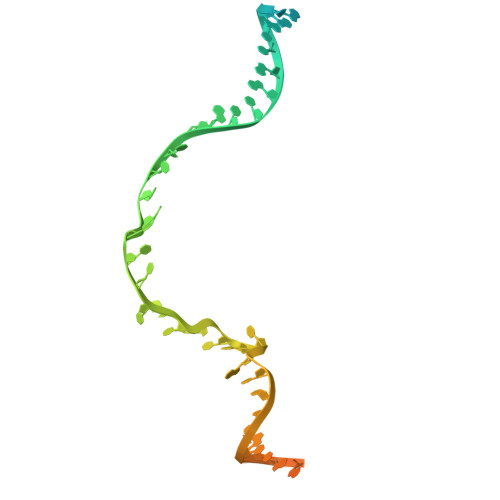
Cryo-electron microscopy visualization of RAD51 filament assembly and end-capping by XRCC3-RAD51C-RAD51D-XRCC2.
Greenhough, L.A., Galanti, L., Liang, C.C., Boulton, S.J., West, S.C.(2025) Science : eaea1546-eaea1546
- PubMed: 41196948
- DOI: https://doi.org/10.1126/science.aea1546
- Primary Citation of Related Structures:
9SVX, 9SVY, 9SW0 - PubMed Abstract:
Homologous recombination repairs DNA double strand breaks and protects stalled replication forks, but how the five RAD51 paralogs contribute to these processes remains unclear. Mutations in the RAD51 paralogs are linked to heritable breast and ovarian cancers and the cancer-prone disease Fanconi anemia. In this work, we show that the RAD51 paralogs assemble into two distinct heterotetrameric complexes, RAD51B-RAD51C-RAD51D-XRCC2 (RAD51B complex) and XRCC3-RAD51C-RAD51D-XRCC2 (XRCC3 complex). The RAD51B complex promotes dynamic adenosine triphosphate hydrolysis-dependent assembly of RAD51 filaments, whereas the XRCC3 complex stably caps the 5'-termini of RAD51 filaments to promote homologous pairing, as visualized by cryo-electron microscopy. Highly conserved across evolution, these complexes reveal insights into RAD51 filament formation and capping during DNA repair and replication fork stabilization.
- DNA Recombination and Repair Laboratory, The Francis Crick Institute, London, UK.
Organizational Affiliation: