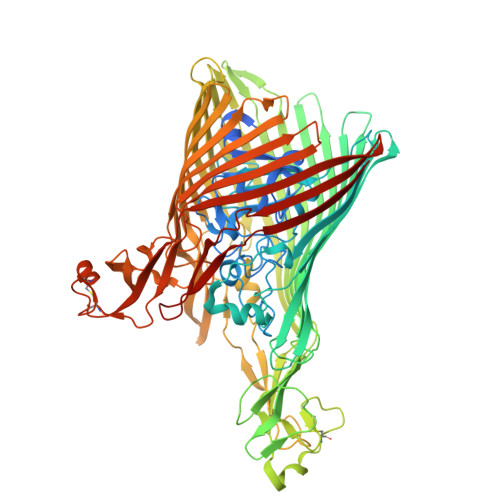
The structure of the bacterial outer membrane transporter FusA enabled by addition of the native lipid lipopolysaccharide.
Machin, J.M., Mosbahi, K., Prakaash, D., Radford, S.E., Walker, D., Kalli, A.C., Ranson, N.A.(2025) J Struct Biol X 12: 100141-100141
- PubMed: 41399487
- DOI: https://doi.org/10.1016/j.yjsbx.2025.100141
- Primary Citation of Related Structures:
9RHR - PubMed Abstract:
Lipopolysaccharide (LPS) is a glycolipid found uniquely in the outer membrane of diderm bacteria, formed of 4-7 acyl chains covalently linked to an extended polysaccharide chain. While a few examples of the interaction between LPS and outer membrane proteins (OMPs) have been structurally characterised, either experimentally or computationally, the precise nature of LPS-OMP interactions and their functional consequences remains unclear. Here, we show that the addition of LPS facilitated cryoEM structure determination of FusA, a 100 kDa TonB-dependent outer membrane transporter from P. atrosepticum . A 2.8 Å structure combined with molecular dynamics of FusA with different LPS models reveals LPS binding sites with a strong LPS interaction site located adjacent to the β-seam region of the FusA β-barrel. The requirement of lipid binding for successful structure determination indicates a stabilisation of the protein, which in turn suggests a potential method for solving other, small OMPs and membrane proteins. Further, it hints at how LPS may mediate protein conformation and thus how LPS and OMPs can work in concert to maintain a structural and functional OM.
- Astbury Centre for Structural Molecular Biology, School of Molecular and Cellular Biology, Faculty of Biological Sciences, University of Leeds, LS2 9JT, United Kingdom.
Organizational Affiliation: