Structure of ARGX-121 Fab fragment in complex with the Fc fragment of IgA1
Voet, S., Provost, M., Deweirdt, L., Verbeiren, J., Gabriels, S., Silence, K., Pannecoucke, E.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ARGX-121 Fab fragment heavy chain | A [auth H], E [auth A] | 244 | Homo sapiens | Mutation(s): 0 | 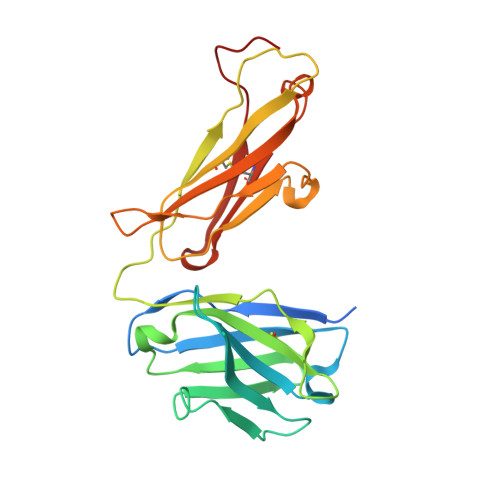 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ARGX-121 Fab fragment light chain | B [auth L], F [auth B] | 235 | Homo sapiens | Mutation(s): 0 | 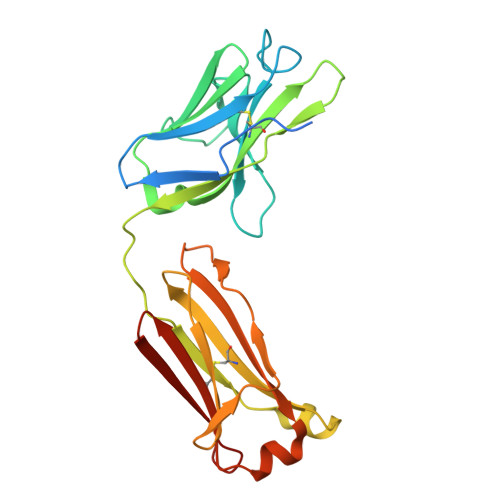 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Isoform 1 of Immunoglobulin heavy constant alpha 1 | C [auth P], D [auth Q] | 213 | Homo sapiens | Mutation(s): 0 Gene Names: IGHA1 | 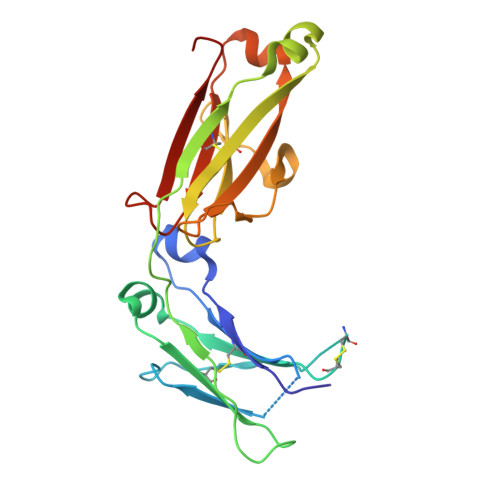 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P01876 (Homo sapiens) Explore P01876 Go to UniProtKB: P01876 | |||||
PHAROS: P01876 GTEx: ENSG00000211895 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P01876 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PEG Query on PEG | I [auth H], J [auth L], M [auth A], O [auth A], P [auth A] | DI(HYDROXYETHYL)ETHER C4 H10 O3 MTHSVFCYNBDYFN-UHFFFAOYSA-N |  | ||
| EDO Query on EDO | G [auth H], H, N [auth A], Q [auth A] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| MG Query on MG | K [auth L], L [auth P], R [auth B] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 77.291 | α = 90 |
| b = 232.312 | β = 90.35 |
| c = 105.519 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| autoPROC | data reduction |
| XDS | data reduction |
| autoPROC | data scaling |
| Aimless | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Not funded | -- |