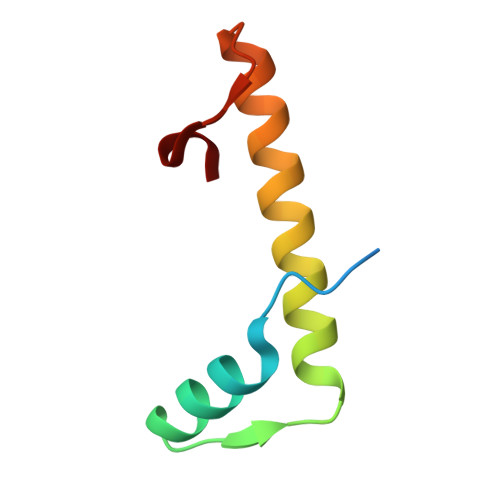
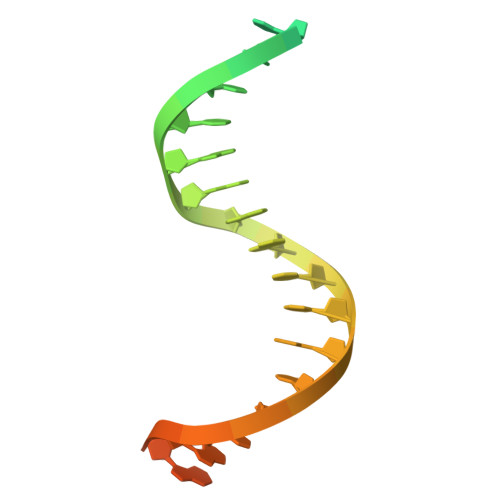
DNA Wrapping by a tetrameric bacterial histone.
Hu, Y., Schwab, S., Qiu, K., Zhang, Y., Bar, K., Reichle, H., Panzera, A., Lupas, A.N., Hartmann, M.D., Dame, R.T., Alva, V., Hernandez Alvarez, B.(2025) Nat Commun 16: 11108-11108
- PubMed: 41381525
- DOI: https://doi.org/10.1038/s41467-025-67425-w
- Primary Citation of Related Structures:
9QT0, 9QT1, 9QT2 - PubMed Abstract:
Histones are conserved DNA-packaging proteins found across all domains of life. In eukaryotes, canonical histones form octamers that wrap ~147 base pairs (bp) of DNA into nucleosomes, while in archaea they form dimers that polymerize into extended hypernucleosomes. Although bacteria were long thought to lack histones, homologs have now been identified in diverse lineages. We previously characterized the histone HBb from Bdellovibrio bacteriovorus, which binds and bends DNA as a dimer. Here, we describe HLp from Leptospira perolatii and show by crystallographic and biophysical analyses that, unlike HBb, it forms stable tetramers and binds DNA nonspecifically, wrapping ~60 bp of DNA around its core. Molecular dynamics simulations, DNA-binding assays, and heterologous expression in Escherichia coli, where HLp reorganizes the nucleoid, support a role in bacterial chromatin organization. These findings expand the repertoire of bacterial histone-DNA interactions and highlight the diversity of histone-based genome organization across the tree of life.
- Department of Protein Evolution, Max Planck Institute for Biology Tübingen, Tübingen, Germany.
Organizational Affiliation: