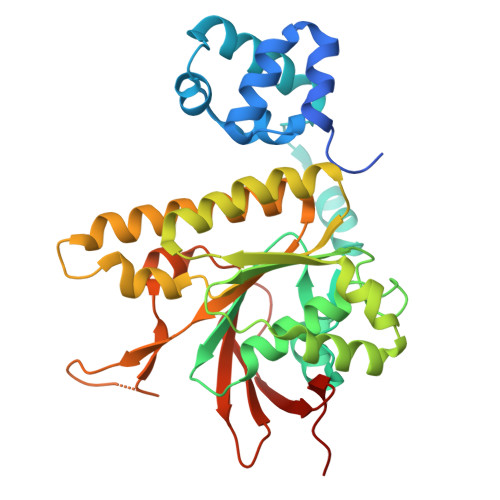
RAD51AP1 is a versatile RAD51 modulator.
Kuhlen, L., Argunhan, B., Liang, P., Zhong, J., Masino, L., Zhang, X.(2025) Proc Natl Acad Sci U S A 122: e2514728122-e2514728122
- PubMed: 41337480
- DOI: https://doi.org/10.1073/pnas.2514728122
- Primary Citation of Related Structures:
9QN8, 9QNA, 9QNB, 9QNC - PubMed Abstract:
RAD51AP1 is an emergent key factor in homologous recombination (HR), the major pathway for accurate repair of DNA double-strand breaks, and in alternative lengthening of telomeres (ALT). Depletion of RAD51AP1 diminishes HR and overexpression is common in cancer, where it is associated with malignancy. Here, we show that RAD51AP1 has a hitherto unknown role in modulating the RAD51 recombinase, the central player in HR. Through a combination of biochemistry and structural biology, we reveal that RAD51AP1 possesses at least three RAD51-binding sites that facilitate its binding across two adjacent RAD51 molecules. We uncover a previously unidentified RAD51-binding mode that stabilizes the RAD51 N-terminal domain and protomer interface in the filaments. We uncover a previously undescribed role for RAD51AP1 in stabilizing RAD51-ssDNA filaments and promoting strand exchange. Our structural data provide the molecular basis for how RAD51AP1 binding induces conformational changes that promote RAD51 DNA association and oligomerization, therefore promoting filament nucleation, stabilization, and strand exchange. Further, we resolved structures of RAD51-ssDNA filaments in the presence of Mg 2+ -ATP and upon hydrolysis to Mg 2+ -ADP, revealing that RAD51 filaments expand upon ATP hydrolysis and explaining how ADP reduces RAD51-DNA binding. Our findings reveal RAD51AP1 as a versatile RAD51 modulator and RAD51 filament remodeler and shed previously unidentified insights into the modulation of HR, which is critical for the maintenance of genome stability.
- Section of Structural and Synthetic Biology, Faculty of Medicine, Imperial College, London SW7 2AZ, United Kingdom.
Organizational Affiliation: