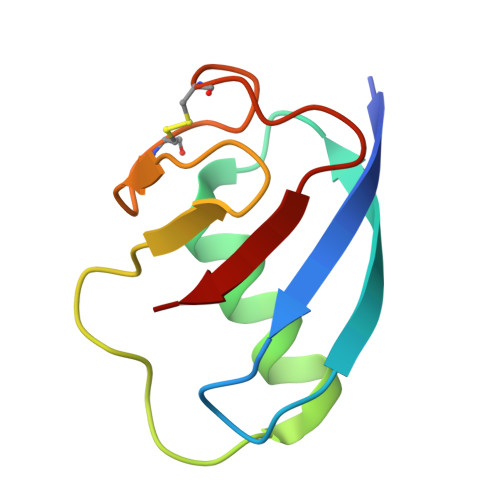
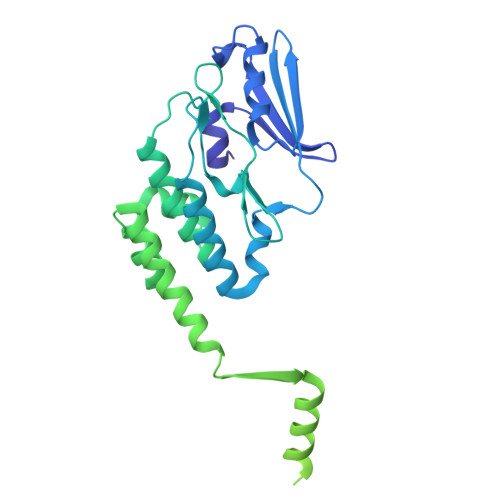
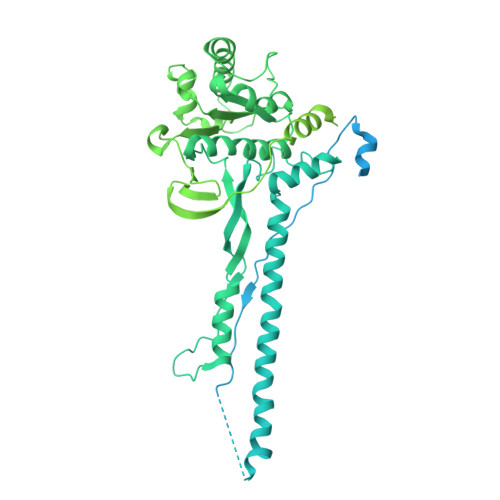
A ubiquitin-like protein controls assembly of a bacterial type VIIb secretion system.
Oka, G.U., Benoit, N., Siroy, A., Gubellini, F., Marza, E., Fronzes, R.(2025) Sci Adv 11: eady9587-eady9587
- PubMed: 41270170
- DOI: https://doi.org/10.1126/sciadv.ady9587
- Primary Citation of Related Structures:
9QKS - PubMed Abstract:
Type VII secretion systems (T7SS) are protein translocation machines crucial for virulence and bacterial competition in Gram-positive bacteria. Despite their importance, the structural basis for assembly of type VIIb secretion systems (T7SSb), a widely distributed variant in Firmicutes, remains poorly understood. We present the cryo-electron microscopy structure of the T7SSb core complex from Bacillus subtilis , revealing how the ubiquitin-like protein YukD, coordinates assembly of the secretion machinery. YukD interacts extensively with the central channel component YukB and facilitates its association with the pseudokinase YukC, forming a stable building block for channel assembly. Time-lapse microscopy and competition assays demonstrate that YukD is essential for proper T7SSb complex formation and contact-dependent bacterial killing. Our findings reveal how bacteria have adapted a ubiquitin-like protein as a structural regulator for assembling a large secretion complex.
- CNRS UMR 5234 Microbiologie Fondamentale et Pathogénicité, Institut Européen de Chimie et Biologie, University of Bordeaux, 33600 Pessac, France.
Organizational Affiliation: