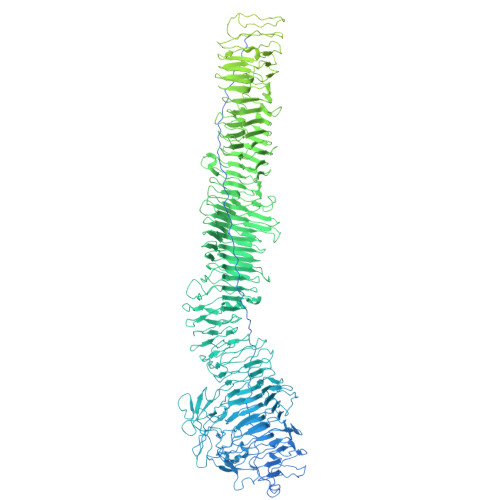
Structural basis of Fusobacterium nucleatum adhesin Fap2 interaction with receptors on cancer and immune cells.
Schopf, F., Marongiu, G.L., Milaj, K., Sprink, T., Kikhney, J., Moter, A., Roderer, D.(2025) Nat Commun 16: 8104-8104
- PubMed: 40883327
- DOI: https://doi.org/10.1038/s41467-025-63451-w
- Primary Citation of Related Structures:
9QE7 - PubMed Abstract:
Fusobacterium nucleatum is overrepresented in the colon microbiome of colorectal cancer patients and has been associated with tumor growth enhancement and metastasis. A pivotal pathogenic factor, the autotransporter adhesin Fap2, facilitates association to cancer and immune cells via the receptors Gal-GalNAc and TIGIT, respectively, leading to deactivation of immune cells. Mechanistic details of the Fap2/TIGIT interaction remain elusive as no structural data are available. Here, we report a system to recombinantly express functional Fap2 on the Escherichia coli surface, which interacts with Gal-GalNAc on cancer cells and with purified TIGIT with submicromolar affinity. Cryo-EM structures of Fap2, alone and in complex with TIGIT, show that the elongated ~50 nm long Fap2 extracellular region binds to TIGIT on its membrane-distal tip via an extension of a β-helix domain. Moreover, by combining structure predictions, cryo-EM, docking and molecular dynamics simulations, we identified a binding pit for Gal-GalNAc on the tip of Fap2.
- Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP), Berlin, Germany.
Organizational Affiliation: