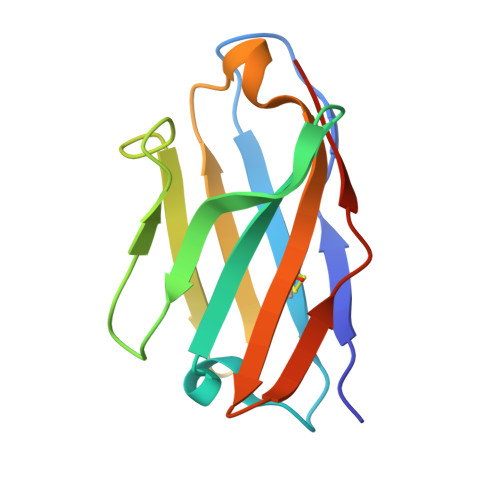
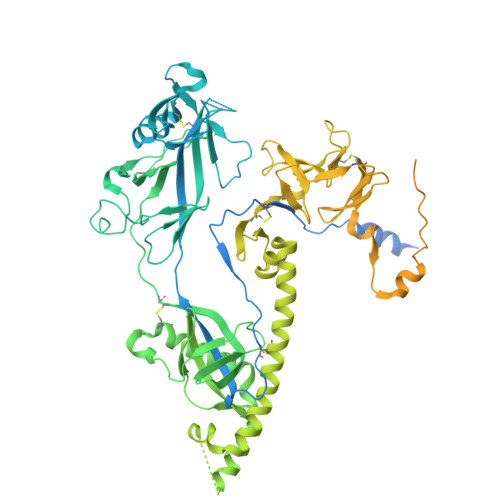
A nanobody specific to prefusion glycoprotein B neutralizes HSV-1 and HSV-2.
Vollmer, B., Ebel, H., Rees, R., Nentwig, J., Mulvaney, T., Schunemann, J., Krull, J., Topf, M., Gorlich, D., Grunewald, K.(2025) Nature 646: 433-441
- PubMed: 40903574
- DOI: https://doi.org/10.1038/s41586-025-09438-5
- Primary Citation of Related Structures:
9IH8, 9Q9L, 9Q9N, 9Q9S - PubMed Abstract:
The nine human herpesviruses, including herpes simplex virus 1 and 2, human cytomegalovirus and Epstein-Barr virus, present a significant burden to global public health 1 . Their envelopes contain at least ten different glycoproteins, which are necessary for host cell tropism, attachment and entry 2 . The best conserved among them, glycoprotein B (gB), is essential as it performs membrane fusion by undergoing extensive rearrangements from a prefusion to postfusion conformation. At present, there are no antiviral drugs targeting gB or neutralizing antibodies directed against its prefusion form, because of the difficulty in structurally determining and using this metastable conformation. Here we show the isolation of prefusion-specific nanobodies, one of which exhibits strong neutralizing and cross-species activity. By mutational stabilization we solved the herpes simplex virus 1 gB full-length prefusion structure, which allowed the bound epitope to be determined. Our analyses show the membrane-embedded regions of gB and previously unresolved structural features 3,4 , including a new fusion loop arrangement, providing insights into the initial conformational changes required for membrane fusion. Binding an epitope spanning three domains, proximal only in the prefusion state, the nanobody keeps wild-type HSV-2 gB in this conformation and enabled its native prefusion structure to be determined. This also indicates the mode of neutralization and an attractive avenue for antiviral interventions.
- Centre for Structural Systems Biology (CSSB), Hamburg, Germany.
Organizational Affiliation: