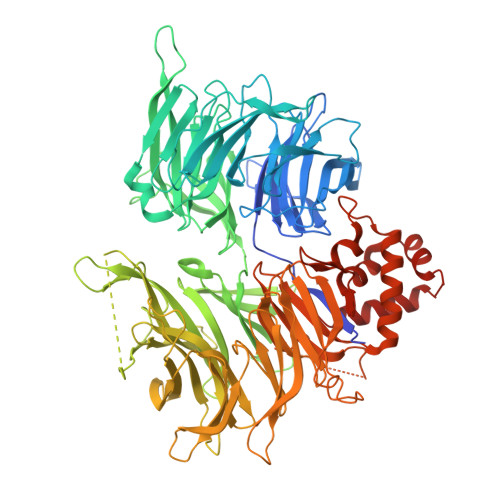
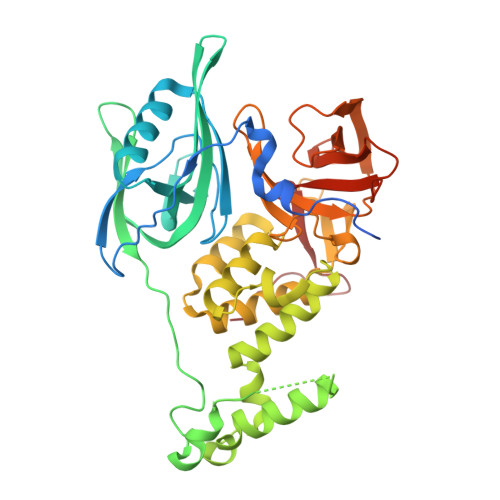
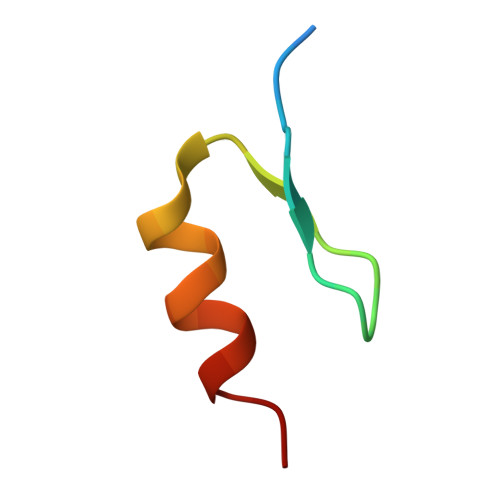
Application of Weighted Interaction-Fingerprints for Rationalizing Neosubstrate Potency and Selectivity of Cereblon-Based Molecular Glues.
Luchini, G., Liu, S., Powers, H.L., Cherney, E., Zhu, J., Danga, K., Thompson, J.W., Shi, L., Pagarigan, B., Wei, D.D., Park, P., Degnan, A.P., Zapf, C.W., Riggs, J.R., Johnson, S., Cummins, T.(2025) J Med Chem 68: 20657-20674
- PubMed: 40994183
- DOI: https://doi.org/10.1021/acs.jmedchem.5c01919
- Primary Citation of Related Structures:
9Q22, 9Q2D - PubMed Abstract:
Cullin-RING Ligase 4 Cereblon (CRL4 CRBN ) (CRBN) E3 ligase modulatory drugs (CELMoDs TM ) make up a successful class of compounds targeting neosubstrates for proteasome-dependent degradation. Early immunomodulatory drugs (IMiDs TM ) target Ikaros and Aiolos degradation. In addition, there are ongoing clinical trials targeting the degradation of biologically relevant proteins such as GSPT1, CK1α, and Helios with CRBN-based molecular glues. To date, most advanced preclinical and clinical CRBN-based molecular glues recruit their neosubstrates through canonical G-motifs, secondary protein features that are structurally similar but have significantly different amino acid sequence identities. Analogous to the development of kinase inhibitors, optimizing both neosubstrate recruitment and degradation selectivity is important to minimize potential off-target activity. Here, we describe a computational structure-based approach to analyze and predict putative ligand interactions important in the neosubstrate ternary complex. This approach provides valuable insights for enhanced designs toward the development of more selective and efficacious CRBN-based molecular glues.
- Discovery & Development Sciences, Bristol Myers Squibb Company, 10300 Campus Point Drive Suite 100, San Diego, California 92121, United States.
Organizational Affiliation: