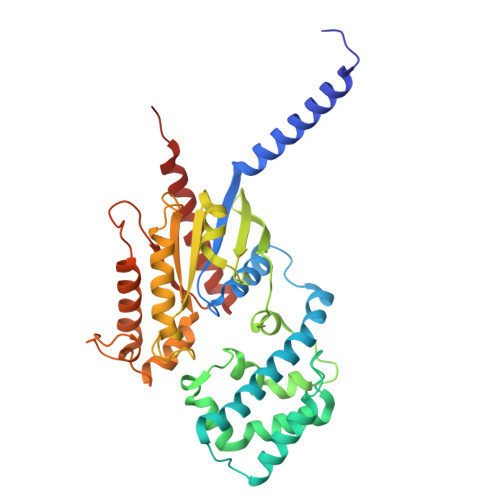
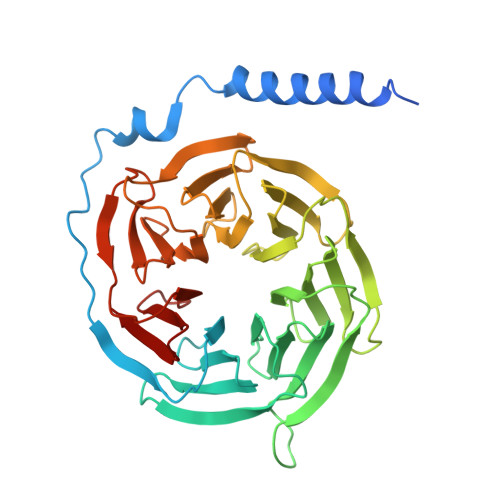
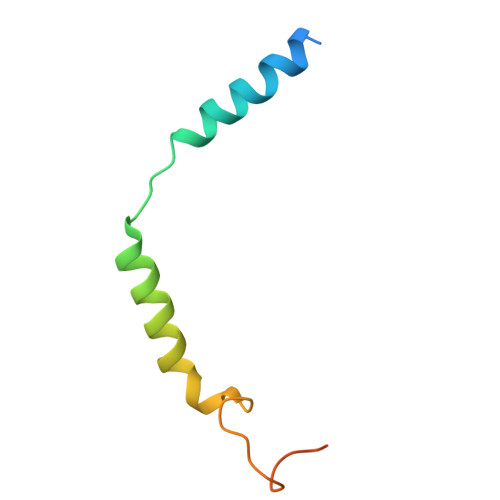
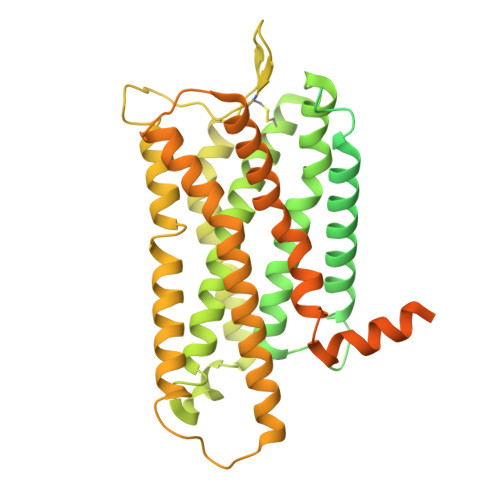
Structural snapshots capture nucleotide release at the mu-opioid receptor.
Khan, S., Tyson, A.S., Ranjbar, M., Zhang, Z., Singh, J., Han, G.W., Gati, C.(2025) Nature 648: 755-763
- PubMed: 41193810
- DOI: https://doi.org/10.1038/s41586-025-09677-6
- Primary Citation of Related Structures:
9PXU, 9PXV, 9PXW, 9PXX, 9PXY, 9PY2, 9PY4 - PubMed Abstract:
As a member of the G protein-coupled receptor superfamily, the μ-opioid receptor (MOR) activates heterotrimeric G proteins by opening the Gα α-helical domain (AHD) to enable GDP-GTP exchange, with GDP release representing the rate-limiting step 1,2 . Here, using pharmacological assays, we show that agonist efficacy correlates with decreased GDP affinity, promoting GTP exchange, whereas antagonists increase GDP affinity, dampening activation. Further investigating this phenomenon, we provide 8 unique structural models and 16 cryogenic electron microscopy maps of MOR with naloxone or loperamide, capturing several intermediate conformations along the activation pathway. These include four GDP-bound states with previously undescribed receptor-G protein interfaces, AHD arrangements and transitions in the nucleotide-binding pocket required for GDP release. Naloxone stalls MOR in a 'latent' state, whereas loperamide promotes an 'engaged' state, which is structurally poised for opening of the AHD domain and subsequent GDP release. These findings, supported by molecular dynamics simulations, identify GDP-bound intermediates and AHD conformations as key determinants of nucleotide exchange rates, providing structural and mechanistic insights into G protein activation and ligand efficacy with broad implications for G protein-coupled receptor pharmacology.
- The Bridge Institute, Michelson Center for Convergent Biosciences, University of Southern California, Los Angeles, CA, USA.
Organizational Affiliation: