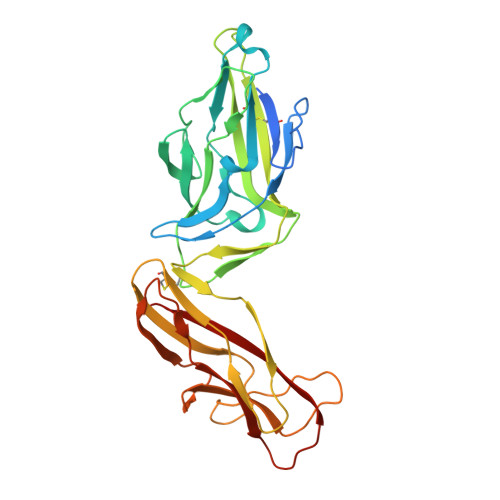
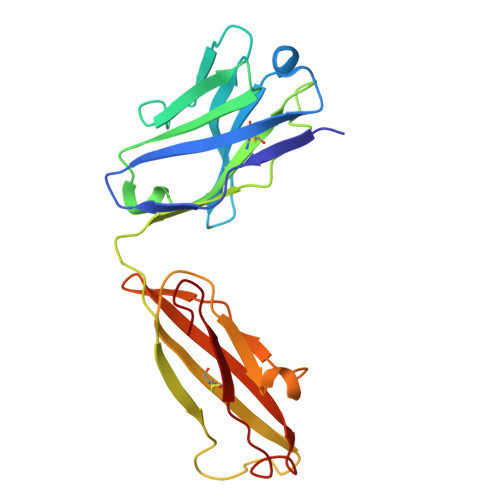
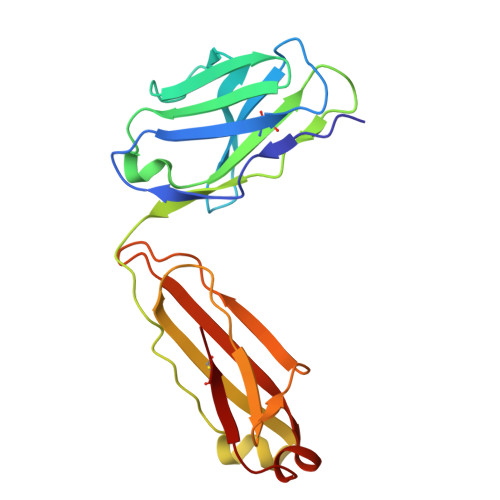
Antibodies disrupt bacterial adhesion by ligand mimicry and allosteric interference.
Hvorecny, K.L., Interlandi, G., Veth, T.S., Aprikian, P., Manchenko, A., Tchesnokova, V.L., Dickinson, M.S., Quispe, J.D., Riley, N.M., Klevit, R.E., Magala, P., Sokurenko, E.V., Kollman, J.M.(2025) Nat Commun 16: 10590-10590
- PubMed: 41298446
- DOI: https://doi.org/10.1038/s41467-025-65666-3
- Primary Citation of Related Structures:
9ME4, 9ME5, 9ME6, 9ME7, 9PTM - PubMed Abstract:
A critical step in infections is the attachment of microorganisms to host cells using lectins that bind glycans, making lectins promising antimicrobial targets. Upon binding mannosylated glycans, FimH, an adhesin in E. coli, undergoes an allosteric transition from an inactive to an active conformation that can act as a catch-bond. Distinct monoclonal antibodies that alter FimH glycan binding are available, but the mechanisms of action remain unclear. Here, we use cryo-electron microscopy, mass spectrometry, adhesion assays, and molecular dynamics simulations to determine the structure-function relationships underlying antibody-FimH binding. Our study demonstrates four mechanisms of action: ligand mimicry by an N-linked, high-mannose glycan; stabilization of the ligand pocket in the inactive state; conformational trapping of the active and inactive states; and locking of the ligand pocket through long-range allosteric effects. These structures reveal multiple mechanisms of antibody responses to an allosteric protein and provide blueprints for antimicrobials that target adhesins.
- Department of Biochemistry, University of Washington, Seattle, WA, USA.
Organizational Affiliation: