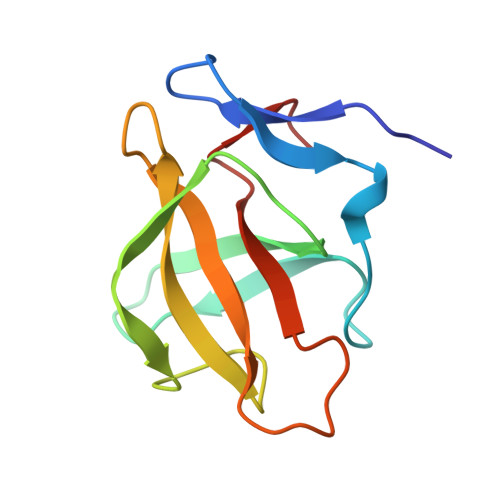
Overcoming CK1 alpha liability in the discovery of a series of isoIndolinone Glutarimides as selective IKZF2 molecular glue degraders.
Zhang, X., Deng, Q., Dhruv, H., Tudor, M., Nagilla, R., Jolivette, L.J., Rice, C.T., Orth, P., Behshad, E., Strickland, C.O., Mohammad, H.P., Sui, Z., Priestley, E.S.(2025) Bioorg Med Chem Lett 124: 130263-130263
- PubMed: 40334997
- DOI: https://doi.org/10.1016/j.bmcl.2025.130263
- Primary Citation of Related Structures:
9O91 - PubMed Abstract:
IKZF2 (Ikaros Family Zinc Finger 2) is a transcription factor implicated in immune regulation and hematologic malignancies, where its dysregulation drives oncogenic programs, immune evasion, and therapy resistance. While targeted protein degradation (TPD) has emerged as a promising strategy, achieving selective IKZF2 degradation remains challenging due to off-target effects on structurally related neosubstrates such as IKZF1/3, SALL4, CK1α, and GSPT1. Here, we report the discovery of a novel series of isoindolinone glutarimide-based molecular glue degraders that selectively degrade IKZF2 while sparing CK1α and other neosubstrates. Through a structure-guided medicinal chemistry campaign, we identified divergent structure-activity relationships (SARs) enabling potent IKZF2 degradation with minimal off-target activity. The lead degrader (31) demonstrated high selectivity between IKZF2 and CK1α with acceptable oral bioavailability in mice. Our findings highlight the feasibility of developing precise IKZF2 degraders and provide a framework for optimizing selectivity in molecular glue design, offering a potential therapeutic strategy for IKZF2-dependent cancers. 2025 Elsevier Ltd. All rights reserved.
- SK Life Sciences Labs, 2500 Renaissance Blvd, King of Prussia, PA 19406, United States. Electronic address: XZhang@sklslabs.com.
Organizational Affiliation: