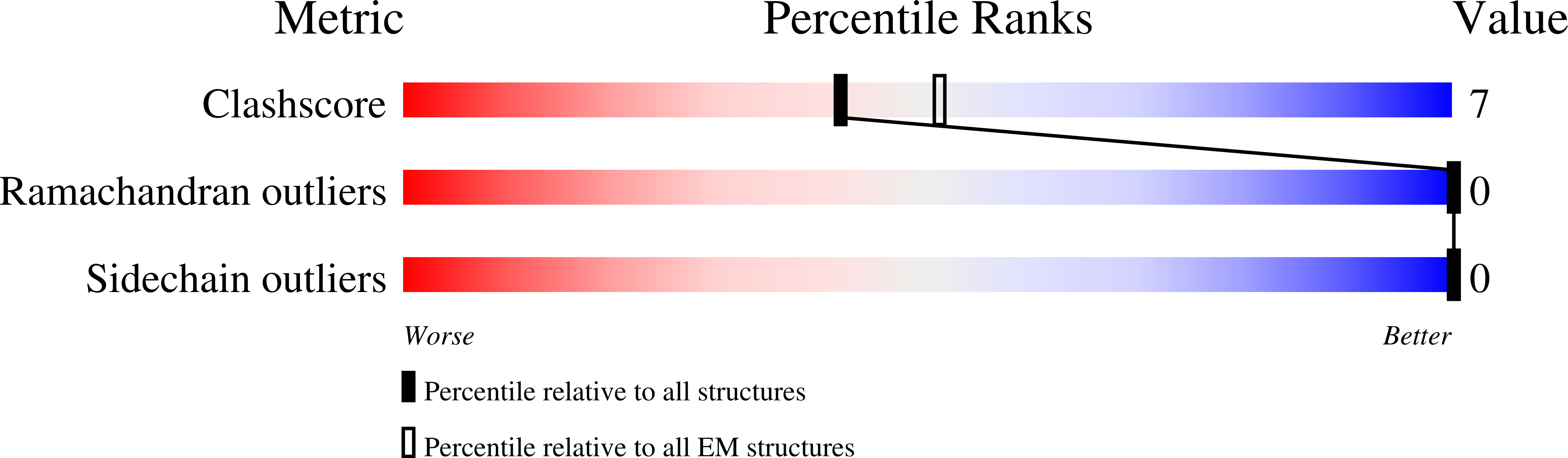Cryo-EM Visualization of Intermolecular pi-Electron Interactions within pi-Conjugated Peptidic Supramolecular Polymers.
Rich-New, S.T., Wang, R., Zia, A., Wang, F., Tovar, J.D.(2025) ACS Macro Lett 14: 1100-1106
- PubMed: 40686463
- DOI: https://doi.org/10.1021/acsmacrolett.5c00360
- Primary Citation of Related Structures:
9O7J, 9O7K, 9O7L - PubMed Abstract:
The self-assembly of "π-peptides" - molecules with π-electron cores substituted with two or more oligopeptide chains - brings organic electronic function into biologically relevant nanomaterials. π-Peptides assemble into fibrillar nanomaterials as driven by enthalpic peptide-based hydrogen bonding networks and pi-core-based quadrupolar interactions. A large body of spectroscopic, morphological and computational studies informs on the nature of the self-assembly process and the resulting nanostructures, but detailed structural information has remained elusive. Inspired by the recent use of cryogenic electron microscopy (cryo-EM) to provide high-resolution structures for synthetic peptide nanomaterials, we present here the use of cryo-EM to offer ca. 3 Å resolution of π-peptide nanomaterial assemblies, visualizing for the first time the nature of the intermolecular π-core electronic interactions responsible for energy transport through these supramolecular materials.
- Department of Biochemistry and Molecular Genetics, University of Alabama at Birmingham, Birmingham, Alabama 35233, United States.
Organizational Affiliation: