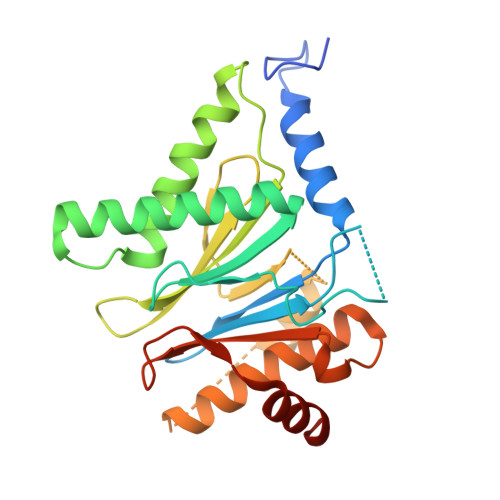
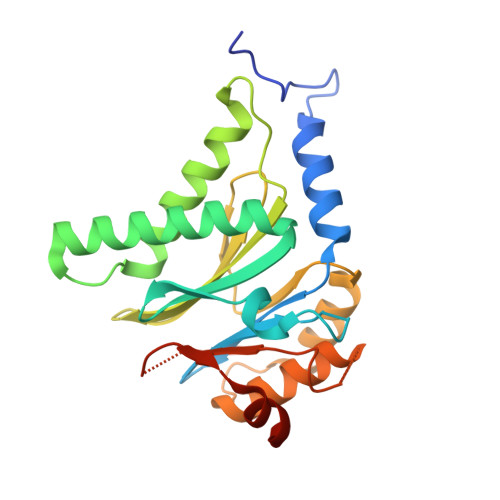
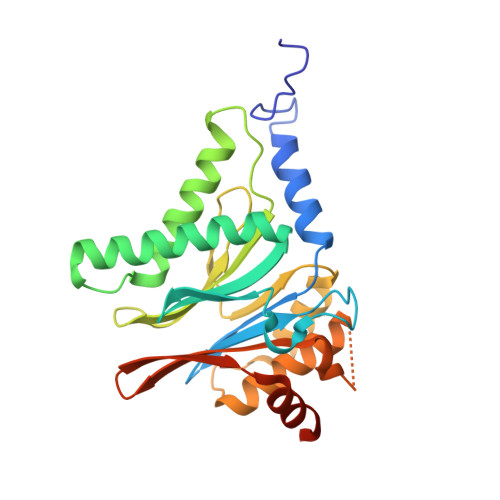
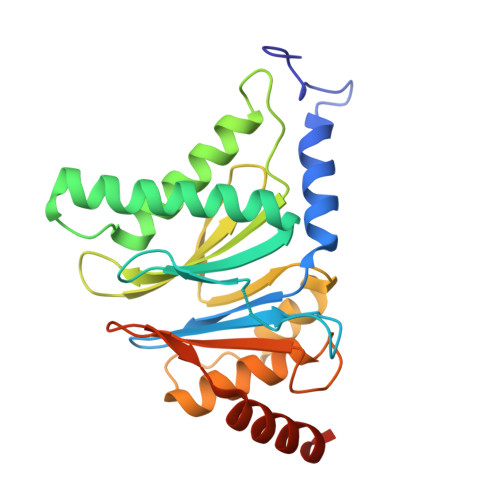
Optimization of Species-Selective Reversible Proteasome Inhibitors for the Treatment of Malaria.
Gahalawat, S., Ray, S., Zhang, X., Deng, X., Han, Y., Chen, Z., Lawong, A., Shackleford, D.M., Katneni, K., Chen, G., Li, P., Ng, A., Zhong, L., Hu, M., McInerney, M., Wang, W., Saunders, J., Collins, D., Jayaseelan, J., Noack, C.L., C Maity, B., De, N., Laleu, B., Campbell, S.F., Phillips, M.A., Charman, S.A., Ready, J.M.(2025) J Med Chem 68: 23485-23520
- PubMed: 41148577
- DOI: https://doi.org/10.1021/acs.jmedchem.5c02394
- Primary Citation of Related Structures:
9O3E, 9O3F - PubMed Abstract:
Malaria remains a critical global health challenge, with increasing resistance to frontline therapies necessitating novel drug targets. The proteasome has emerged as a promising target for antimalarial drug discovery. This study describes efforts to optimize a series of species-selective reversible inhibitors targeting the Plasmodium falciparum 20S proteasome. Starting from the carboxypiperidine scaffold identified through a high-throughput viability screen, we conducted iterative structure-activity relationship studies, leading to the development of highly potent and selective inhibitors with good oral bioavailability. Lead compounds demonstrated nanomolar potency against P. falciparum blood-stage parasites and selective inhibition of the parasite proteasome over the human counterpart. Cryo-EM structural studies confirmed binding at the β5 subunit, while in vivo pharmacokinetic studies identified promising candidates for further development. These findings support proteasome inhibition as a viable strategy for novel antimalarial drug development.
- Department of Biochemistry, UT Southwestern Medical Center, Dallas, Texas 75390, United States.
Organizational Affiliation: