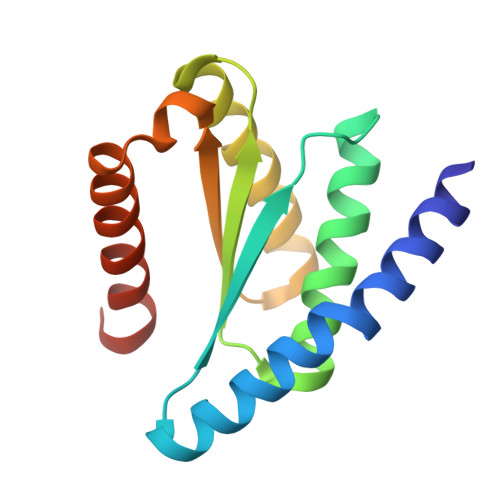
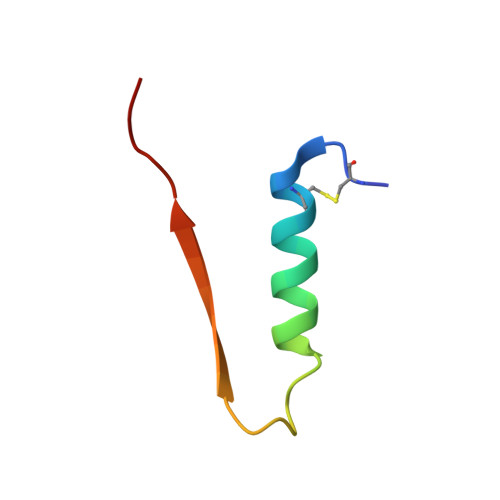
Diffusing protein binders to intrinsically disordered proteins.
Liu, C., Wu, K., Choi, H., Han, H.L., Zhang, X., Watson, J.L., Ahn, G., Zhang, J.Z., Shijo, S., Good, L.L., Fischer, C.M., Bera, A.K., Kang, A., Brackenbrough, E., Coventry, B., Hick, D.R., Qamar, S., Li, X., Decarreau, J., Gerben, S.R., Yang, W., Goreshnik, I., Vafeados, D., Wang, X., Lamb, M., Murray, A., Kenny, S., Bauer, M.S., Hoofnagle, A.N., Zhu, P., Knowles, T.P.J., Baker, D.(2025) Nature 644: 809-817
- PubMed: 40739343
- DOI: https://doi.org/10.1038/s41586-025-09248-9
- Primary Citation of Related Structures:
9CC5, 9CC6, 9NZH - PubMed Abstract:
Proteins that bind to intrinsically disordered proteins (IDPs) and intrinsically disordered regions (IDRs) with high affinity and specificity could be useful for therapeutic and diagnostic applications 1-4 . However, a general methodology for targeting IDPs or IDRs has yet to be developed. Here we show that starting only from the target sequence of the input, and freely sampling both target and binding protein conformations, RFdiffusion 5 can generate binders to IDPs and IDRs in a wide range of conformations. We used this approach to generate binders to the IDPs amylin, C-peptide, VP48 and BRCA1_ARATH in diverse conformations with a dissociation constant (K d ) ranging from 3 to 100 nM. For the IDRs G3BP1, common cytokine receptor γ-chain (IL-2RG) and prion protein, we diffused binders to β-strand conformations of the targets, obtaining K d between 10 and 100 nM. Fluorescence imaging experiments show that the binders bind to their respective targets in cells. The G3BP1 binder disrupts stress granule formation in cells, and the amylin binder inhibits amyloid fibril formation and dissociates existing fibres, enables targeting of both monomeric and fibrillar amylin to lysosomes, and increases the sensitivity of mass spectrometry-based amylin detection. Our approach should be useful for creating binders to flexible IDPs or IDRs spanning a wide range of intrinsic conformational preferences.
- Department of Biochemistry, University of Washington, Seattle, WA, USA.
Organizational Affiliation: