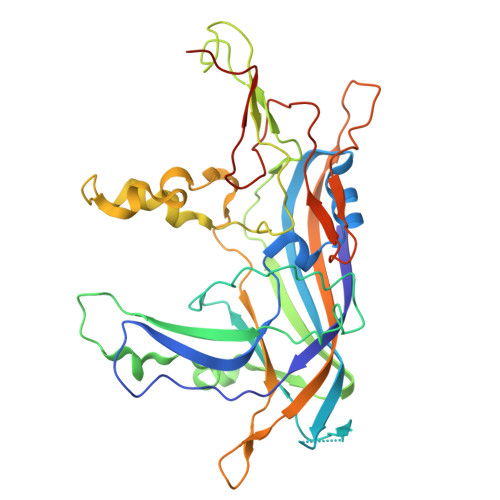
Capsid Structure of the Fish Pathogen Syngnathus Scovelli Chapparvovirus Offers a New Perspective on Parvovirus Structural Biology.
Penzes, J.J., Kaelber, J.T.(2025) Viruses 17
- PubMed: 40431691
- DOI: https://doi.org/10.3390/v17050679
- Primary Citation of Related Structures:
9NEK - PubMed Abstract:
Chapparvoviruses (ChPVs) comprise a divergent lineage of the Parvoviridae ssDNA virus family and evolved to infect vertebrate animals independently from the Parvovirinae subfamily. Despite being pathogenic and widespread in environmental samples and metagenomic assemblies, their structural characterization has proven challenging. Here, we report the first structural analysis of a ChPV, represented by the fish pathogen, Syngnathus scovelli chapparvovirus (SsChPV). We show through the SsChPV structure that the lineage harbors a surface morphology, subunit structure, and multimer interactions that are unique among parvoviruses. The SsChPV capsid evolved a threefold-related depression of α-helices that is analogous to the β-annulus pore of denso- and hamaparvoviruses and may play a role in monomer oligomerization during assembly. As interacting β-strands are absent from the twofold symmetry axis, the viral particle lacks the typical stability and resilience of parvovirus capsids. Although all parvoviruses thus far rely on the threading of large, flexible N-terminal domains to the capsid surface for their intracellular trafficking, our results show that ChPVs completely lack any such N-terminal sequences. This led to the subsequent degradation of their fivefold channel, the site of N-terminus externalization. These findings suggest that ChPVs harbor an infectious pathway that significantly deviates from the rest of the Parvoviridae .
- Institute for Quantitative Biomedicine, Rutgers, The State University of New Jersey, Piscataway, NJ 08812, USA.
Organizational Affiliation: