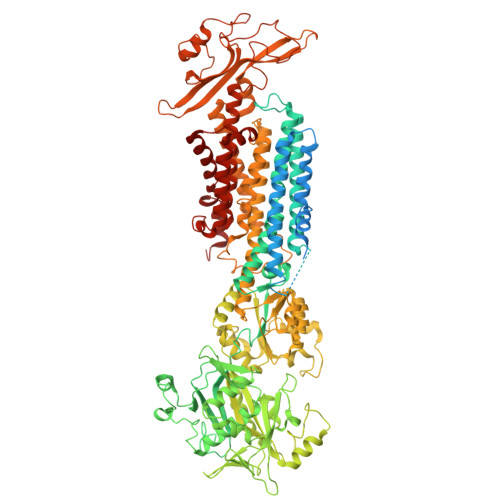
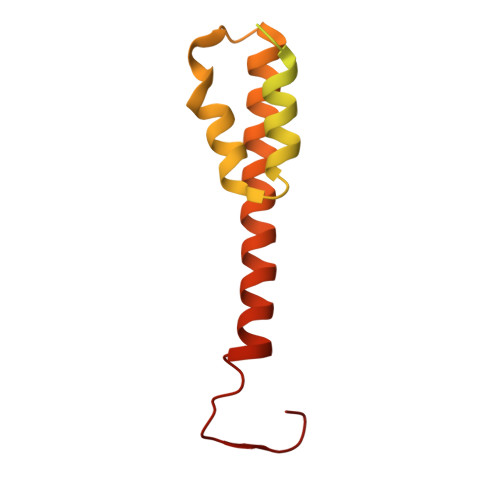
Endogenous structure of antimalarial target PfATP4 reveals an apicomplexan-specific P-type ATPase modulator.
Haile, M.T., Shukla, A., Zhen, J., Mather, M.W., Bhatnagar, S., Morrisey, J.M., Zhang, Z., Vaidya, A.B., Ho, C.M.(2025) Nat Commun 16: 9092-9092
- PubMed: 41115914
- DOI: https://doi.org/10.1038/s41467-025-64815-y
- Primary Citation of Related Structures:
9N10 - PubMed Abstract:
The Plasmodium falciparum sodium efflux pump PfATP4 is a leading antimalarial target, but suffers from a lack of high-resolution structural information needed to identify functionally important features in conserved regions and guide rational design of next generation inhibitors. Here, we determine a 3.7 Å cryoEM structure of PfATP4 purified from CRISPR-engineered P. falciparum parasites, revealing a previously unknown, apicomplexan-specific binding partner, PfABP, which forms a conserved, likely modulatory interaction with PfATP4. The discovery of PfABP presents an unexplored avenue for designing PfATP4 inhibitors.
- Department of Microbiology and Immunology, Columbia University Irving Medical Center, New York, NY, USA.
Organizational Affiliation: