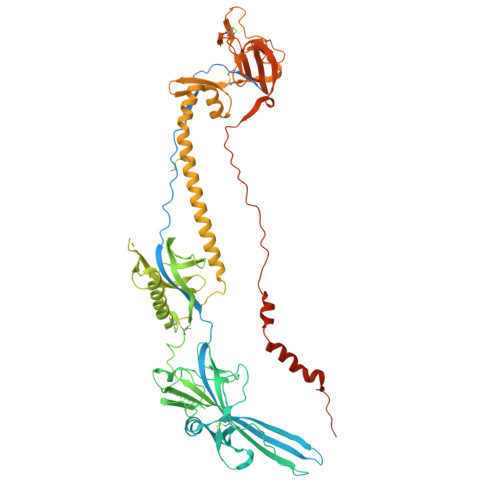
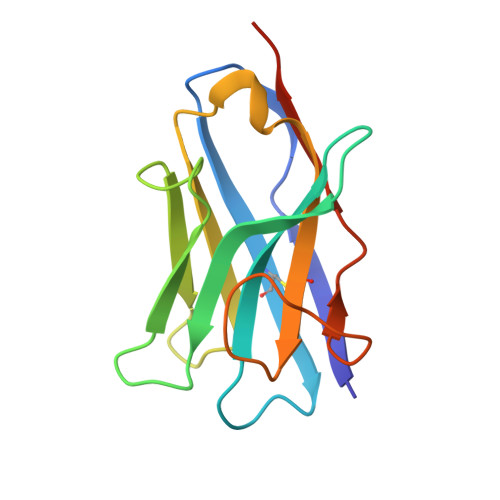
Identification and engineering of potent bispecific antibodies that protect against herpes simplex virus recurrent disease.
Lee, C.V., Viadiu, H., Kalamkar, A., Bernstein, D.I., Pae, A., Yu, X., Wong, S., Bravo, F.J., Ding, S., Seto, E., Hung, M., Yu, Y., Xing, W., Papalia, G.A., Kan, W., Carr, B., Thomas, M., Tong, L., Desai, P., Jarrousse, N., Mercier, A., Holdorf, M.M., Fletcher, S.P., Abernathy, E.(2025) Cell Rep 44: 116063-116063
- PubMed: 40716063
- DOI: https://doi.org/10.1016/j.celrep.2025.116063
- Primary Citation of Related Structures:
9MVU, 9MW5, 9MY8 - PubMed Abstract:
Herpes simplex virus (HSV) causes lifelong infections, including oral and genital herpes. There is no vaccine, and current antivirals are only partially effective at reducing symptoms and transmission. Therapeutic antibodies offer a potentially long-acting treatment option, although efforts to pursue this have been limited. We performed an alpaca immunization campaign and discovered high-affinity antibodies that both neutralized and completely blocked cell-to-cell spread (CCS), a key mechanism by which HSV evades neutralizing antibodies. Unexpectedly, we found that engineering antibodies into a bispecific format targeting two viral glycoproteins dramatically increased antiviral potency. Solving the structures of three antibodies using cryo-electron microscopy (cryo-EM) revealed a mechanistic understanding of how the bispecific format could enhance potency. Lastly, these bispecific antibodies significantly reduced lesion development in the guinea pig model of genital herpes, demonstrating that delayed dosing after latency establishment can reduce disease and confirming their potential as a transformative treatment option.
- Gilead Sciences, Inc., Foster City, CA, USA.
Organizational Affiliation: