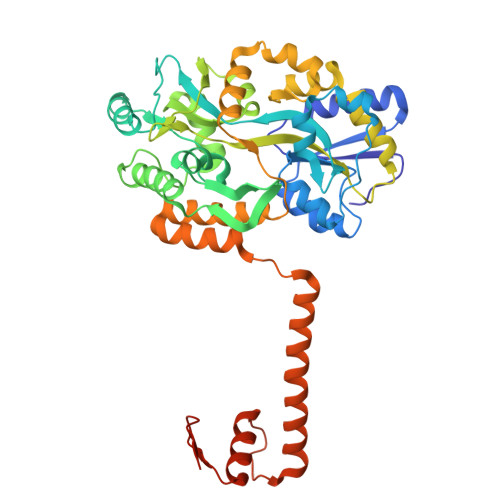
Single cis-elements in brassinosteroid-induced upregulated genes are insufficient to recruit both redox states of the BIL1/BZR1 DNA-binding domain.
Nosaki, S., Ohtsuka, M., Nakano, T., Tanokura, M., Miyakawa, T.(2025) FEBS Lett 599: 3369-3380
- PubMed: 40882013
- DOI: https://doi.org/10.1002/1873-3468.70147
- Primary Citation of Related Structures:
9M73, 9M74 - PubMed Abstract:
The plant-specific transcription factor BRZ-INSENSITIVE-LONG 1 (BIL1)/BRASSINAZOLE-RESISTANT 1 (BZR1) regulates the growth of Arabidopsis thaliana via brassinosteroid signaling, acting as both a gene repressor and activator. Its upregulation requires environmental cues mediated by PHYTOCHROME INTERACTING FACTOR 4 (PIF4), with oxidative modifications via H 2 O 2 enhancing their interaction. However, the nature of the tripartite complex of cis-elements, BIL1/BZR1, and PIF4 under redox changes remains unclear. Here, we demonstrate that oxidation of the DNA-binding domain (DBD) of BIL1/BZR1 alters its DNA-binding ability. However, single cis-elements enriched in brassinosteroid-induced genes do not support binding of either redox form, nor does BIL1/BZR1 DBD heterodimerize with PIF4 DBD on these elements. These findings highlight the complexity of brassinosteroid transcriptional regulation beyond DNA-binding specificity and redox modifications.
- Institute of Life and Environmental Sciences, University of Tsukuba, Japan.
Organizational Affiliation: