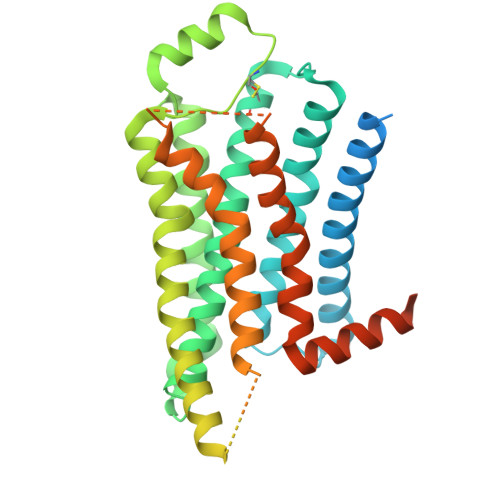
Structural insights into endogenous agonist selectivity of aminergic receptors from the octopamine beta 2 receptor.
Hori, T., Katsura, K., Miyamoto-Kohno, S., Uchikubo-Kamo, T., Yonemochi, M., Shirouzu, M.(2025) PNAS Nexus 4
- PubMed: 41393170
- DOI: https://doi.org/10.1093/pnasnexus/pgaf376
- Primary Citation of Related Structures:
9M55, 9M57 - PubMed Abstract:
Tyrosine-derived amines (TDAs), such as octopamine, noradrenaline, dopamine, and tyramine, are essential neurotransmitters that play diverse roles in various physiological processes. The distinct receptor selectivity of these structurally similar molecules is vital for their specific functions. However, all these receptors belong to the same subfamily of G-protein-coupled receptors and share high sequence homology within their orthosteric binding sites. The molecular basis of this selectivity remains unclear because of the absence of structural data on octopamine and tyramine receptors. In this study, we present cryo-electron microscopy structures of the deer tick octopamine β 2 receptor (octβ 2 R) bound to octopamine or N -2,4-dimethylphenyl- N '-methylformamidine (DPMF), an acaricidal amitraz metabolite. Octopamine and DPMF formed aromatic interactions with Y307 6.55 and F328 7.39 , residues crucial for octβ 2 R activation. The lower potency of other TDAs for octβ 2 R stems from the subtle effect of functional groups on both interactions, i.e. the meta-hydroxyl group of noradrenaline and dopamine hinders edge-to-edge interaction with Y307 6.55 , and the absence of a 1-hydroxyl group in dopamine and tyramine prevents π-hydrogen bonding with F328 7.39 . These structural insights into octβ 2 R selectivity are likely applicable across other TDA receptors, highlighting the pivotal role of residues 6.55 and 7.39. Consequently, the elucidated selection mechanism provides fundamental knowledge of aminergic ligand recognition, a process crucial for neurotransmission and overall organismal function.
- Laboratory for Protein Functional and Structural Biology, RIKEN Center for Integrative Medical Sciences, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama, Kanagawa 230-0045, Japan.
Organizational Affiliation: