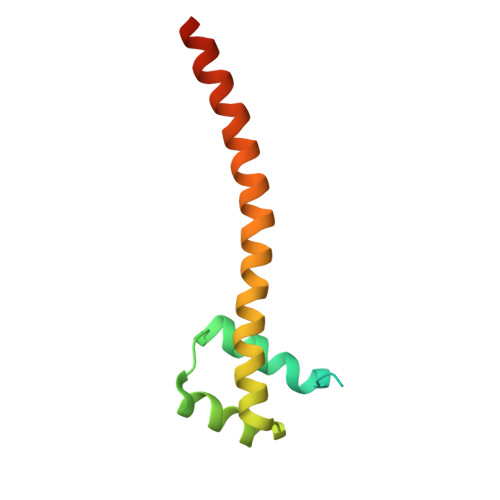
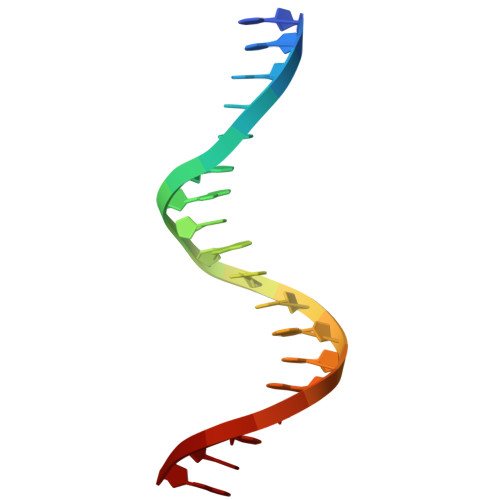
Molecular insights into DNA recognition by HD-Zip transcription factors.
Chen, W., Yan, W., Jiang, K., Huang, H.(2025) Plant Physiol 198
- PubMed: 40583771
- DOI: https://doi.org/10.1093/plphys/kiaf292
- Primary Citation of Related Structures:
9LPE - PubMed Abstract:
Homeodomain-leucine zipper (HD-Zip) genes encode a large family of plant-specific transcription factors (TFs) that are integral to plant development, growth, regulation, and responses to environmental and hormonal signals. While the roles and mechanisms of HD-Zip TFs have been extensively studied, the structural basis for their DNA recognition remains unclear. In this study, we analyzed DAP-seq data and identified consensus DNA motifs, 5'-AAT[W]AT-3' and 5'-[N]AAA[N][N]-3', preferentially bound by HD-Zip TFs. Both motifs feature a 5'-AA(T/A)-3' core, which is shared across previously identified HD-Zip target sequences, suggesting a common recognition feature within the HD-Zip family. Focusing on the well-characterized HD-Zip IV TF PROTEIN PRODUCTION FACTOR 2 (PDF2) from Arabidopsis (Arabidopsis thaliana) and its interaction with the L1 box DNA sequence, our structural and biochemical analyses revealed that the PDF2 HD-ZA module forms a dimer to specifically recognize the 5'-AATG-3' core through an asymmetric binding mode. In this mode, only the primary recognition helix of 1 protomer and the N-arm of the other protomer in the PDF2 HD-ZA dimer are involved in specific DNA interactions. Our study offers insights into the molecular mechanisms of HD-Zip TFs and provides a structural template for engineering applications in agricultural research.
- Institute for Biological Electron Microscopy, Key Laboratory of Molecular Design for Plant Cell Factory of Guangdong Higher Education Institutes, Department of Chemical Biology, School of Life Sciences, Southern University of Science and Technology, Shenzhen 518055, China.
Organizational Affiliation: