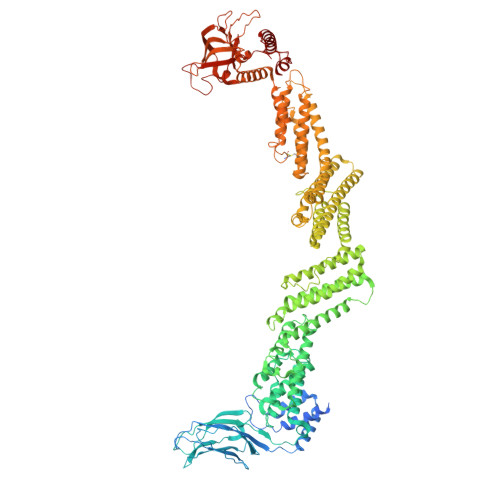
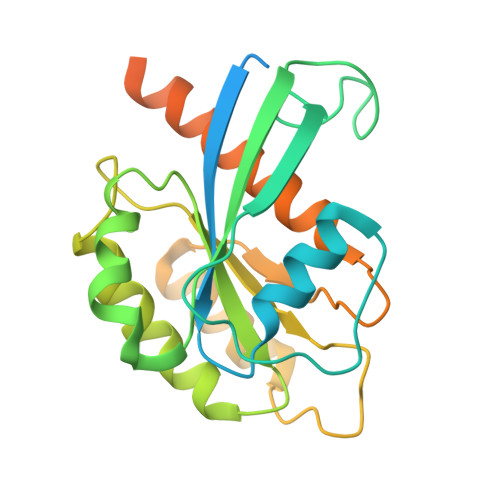
Munc13-4 mediates tumor immune evasion by regulating the sorting and secretion of PD-L1 via exosomes.
Liu, C., Liu, D., Zheng, X., Guan, J., Zhou, X., Zhang, H., Wang, S., Li, Q., Lu, G., He, J., Ma, C.(2025) Nat Commun 16: 9080-9080
- PubMed: 41083534
- DOI: https://doi.org/10.1038/s41467-025-64149-9
- Primary Citation of Related Structures:
9LA9 - PubMed Abstract:
Tumor-derived exosomes carry programmed death-ligand 1 (PD-L1), which binds programmed cell death protein 1 (PD-1) on T cells, suppressing immune responses locally and systemically. However, the mechanisms governing exosomal PD-L1 sorting and secretion remain elusive. Here, we identify Munc13-4 as a crucial regulator of this process. Deletion of Munc13-4 in breast tumors enhances T cell-mediated anti-tumor immunity, suppresses tumor growth, and improves the efficacy of immune checkpoint inhibitors. Mechanistically, Munc13-4 collaborates with hepatocyte growth factor-regulated tyrosine kinase substrate (HRS), Rab27, and SNAREs to facilitate PD-L1 sorting and secretion via exosomes. Cryogenic electron microscopy (cryo-EM) analysis of the Munc13-4-Rab27a complex provide structural insights into exosome secretion. Importantly, PD-L1 sorting relies on a ternary complex composed of Munc13-4, PD-L1 and HRS, which is regulated by interferon gamma (IFNγ) signaling. A designed peptide that disrupts Munc13-4-PD-L1 interaction impedes PD-L1 sorting, enhances antitumor immunity, and suppresses tumor growth, highlighting the therapeutic potential of targeting this pathway.
- Key Laboratory of Molecular Biophysics of the Ministry of Education, College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, China.
Organizational Affiliation: