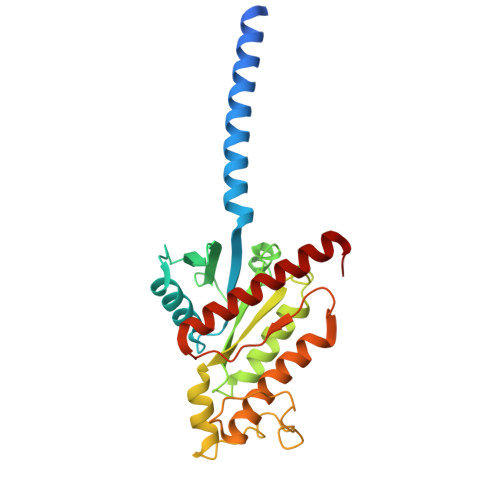
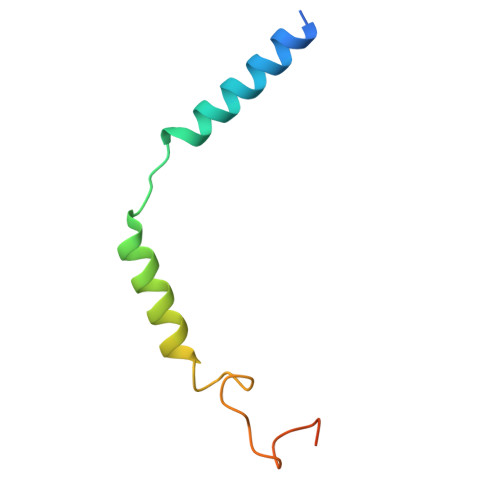
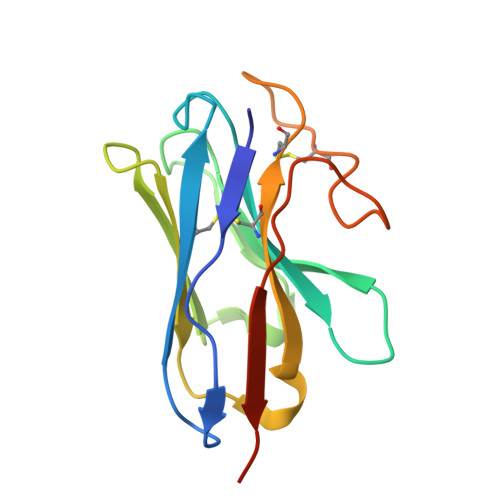
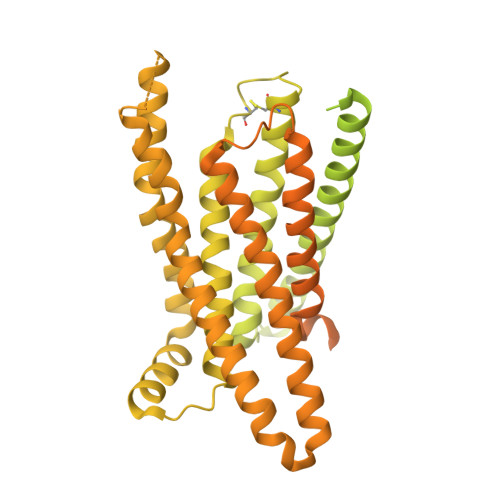
Structural basis of beta-glucopyranoside salicin recognition by a human bitter taste GPCR.
Wang, X., Zhou, C., Ao, W., Wu, L., Wu, Y., Xu, W., Liu, S., Tan, Q., Wang, L., Zhao, F., Liu, J., Pei, Y., Zhao, S., Hua, T.(2025) Cell Rep 44: 115604-115604
- PubMed: 40261795
- DOI: https://doi.org/10.1016/j.celrep.2025.115604
- Primary Citation of Related Structures:
9K6L, 9KPD, 9KPE, 9KPF - PubMed Abstract:
The human perception of bitterness is mediated by type 2 taste receptors (TAS2Rs), which recognize a broad array of bitter substances with distinct chemical properties. TAS2R16 exhibits a pronounced selectivity for β-glucoside-moiety-containing compounds, such as salicin from willow bark. However, the molecular mechanism of moiety-specific recognition and receptor activation in TAS2R16 remains unclear. Here, we present cryoelectron microscopy structures of the salicin-activated human TAS2R16 complexed with gustducin and G i1 and G i2 proteins. The binding mode of salicin with TAS2R16 and the specific interactions of the β-D-glucopyranoside moiety are detailed. Together with molecular docking and mutagenesis data, this study uncovers the structural underpinnings of TAS2R16's group-specific recognition, receptor activation, and subsequent gustducin and G i protein coupling. These findings advance our understanding of human bitter taste receptors and provide a foundation for structural modifications of bitter glycosides, opening potential therapeutic applications.
- iHuman Institute, ShanghaiTech University, Shanghai 201210, China; School of Life Science and Technology, ShanghaiTech University, Shanghai 201210, China.
Organizational Affiliation: