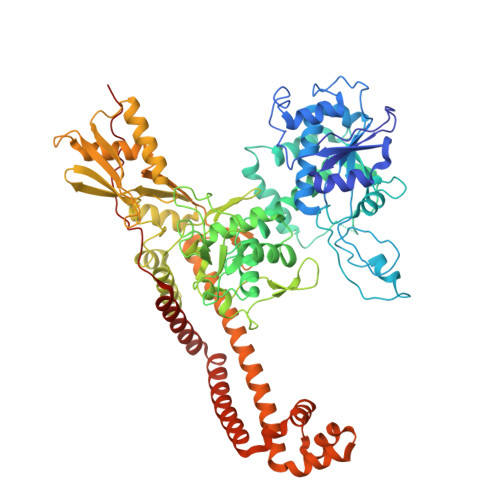
Structural interactions of BWC0977 with Klebsiella pneumoniae topoisomerase IV and biochemical basis of its broad-spectrum activity.
Nandishaiah, R., Murakami, S., Hameed P, S., Aoki, M., Okada, U., Yamashita, E., Venkatesan, S., Bharatham, N., Sarma, S., Shanbhag, A.P., Sharma, S., Rao, R., Ramachandran, V., Balasubramanian, V., Datta, S., Katagihallimath, N.(2025) Commun Biol 8: 1666-1666
- PubMed: 41291128
- DOI: https://doi.org/10.1038/s42003-025-09055-y
- Primary Citation of Related Structures:
9KGT - PubMed Abstract:
Antimicrobial resistance is a growing global health crisis driving the urgent need for effective broad-spectrum antibiotics. BWC0977 is a pyrazino-oxazinone based novel bacterial topoisomerase inhibitor (NBTI) currently in Phase 1 clinical trials and demonstrates potent activity against multidrug-resistant Gram-negative and Gram-positive bacteria. It targets both DNA gyrase and topoisomerase IV with balanced low-nanomolar potencies, showing remarkable superiority over ciprofloxacin and gepotidacin. We report the first 3.05 Å cocrystal structure of BWC0977 bound to Klebsiella pneumoniae topoisomerase IV, revealing its binding mode and interaction residues. The reduced inhibition of BWC0977 against purified gyrase enzymes carrying an individual mutation at these residues supports the relevance of these molecular interactions. Mutational analyses in Escherichia coli strains show that single target mutations in gyrA or parC do not confer resistance, while simultaneous mutations in both genes result in over 250-fold reduced susceptibility. The compound also demonstrates more than 5000-fold selectivity for bacterial over human topoisomerases and retains efficacy against fluoroquinolone and carbapenem-resistant clinical isolates. Together, these structural, biochemical, and microbiological insights elucidate BWC0977's broad-spectrum antibacterial activity and reduced vulnerability to resistance, establishing it as a promising next-generation antibiotic to address the global threat of antimicrobial resistance.
- Bugworks Research India Pvt. Ltd., Center for Cellular & Molecular Platforms, National Center for Biological Sciences, Bangalore, India.
Organizational Affiliation: