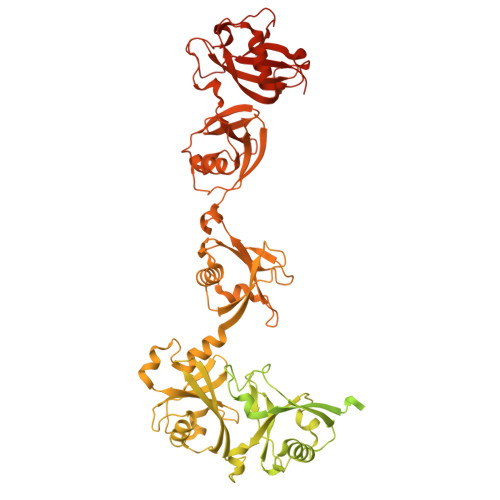
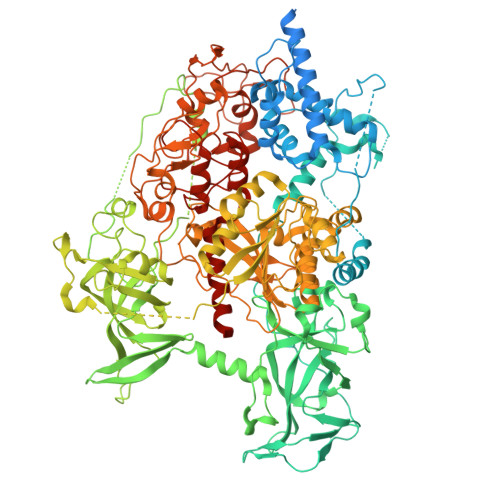
Structures of USP7 in active and inactive states bound to DNMT1 revealed by cryo-EM.
Nakamura, N., Yoshimi, S., Kikuchi, A., Onoda, H., Kori, S., Nakanishi, M., Nishiyama, A., Arita, K.(2025) Structure 33: 1510-1518.e5
- PubMed: 40645181
- DOI: https://doi.org/10.1016/j.str.2025.06.005
- Primary Citation of Related Structures:
9K2W, 9K2X - PubMed Abstract:
The ubiquitin signal generated by UHRF1 is essential for DNA methylation maintenance by recruiting DNA methyltransferase 1 (DNMT1) to hemimethylated DNA through strong binding of its replication foci targeting sequence (RFTS) domain to ubiquitinated histone H3. The ubiquitin-specific protease 7 (USP7) forms a complex with DNMT1 and removes ubiquitin from H3. However, it remains unknown how USP7 deubiquitinates ubiquitinated H3 upon strong binding of the DNMT1 RFTS domain. Here, we show the activation mechanism of USP7 by combining biochemical and structural studies. USP7 is inactive toward ubiquitinated H3 in complex with the RFTS domain. However, when complexed with DNMT1, USP7 efficiently deubiquitinates ubiquitinated H3. Cryogenic electron microscopy (cryo-EM) single particle analysis revealed that USP7 bound to DNMT1 undergoes an open (inactive) and closed (active) conformational transition. Our findings provide mechanistic insights into the activation of USP7 upon binding to DNMT1 and contribute to a better understanding of the deubiquitination process in DNA methylation maintenance.
- Structural Biology Laboratory, Graduate School of Medical Life Science, Yokohama City University, 1-7-29, Suehiro-cho, Tsurumi-ku, Yokohama 230-0045, Kanagawa, Japan.
Organizational Affiliation: