Elucidating the Mechanism Underlying UBA7-UBE2L6 Disulfide Complex Formation.
Chen, P.-T., Yeh, J.-Y., Weng, J.-H., Wu, K.-P.(2025) Elife
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(2025) Elife
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| UBA7 protein | 998 | Bos taurus | Mutation(s): 0 Gene Names: UBA7 | 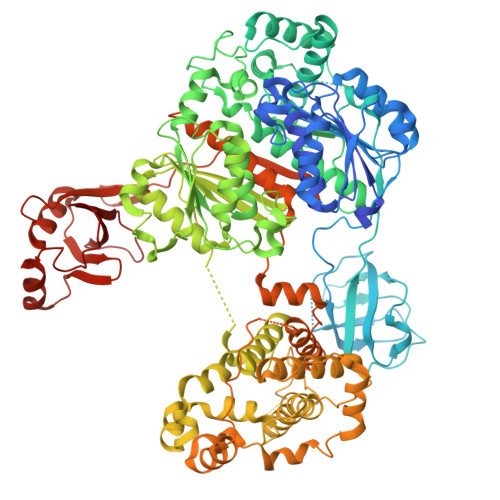 | |
UniProt | |||||
Find proteins for Q5GF34 (Bos taurus) Explore Q5GF34 Go to UniProtKB: Q5GF34 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q5GF34 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ubiquitin/ISG15-conjugating enzyme E2 L6 | 159 | Bos taurus | Mutation(s): 0 Gene Names: UBE2L6 EC: 2.3.2.23 | 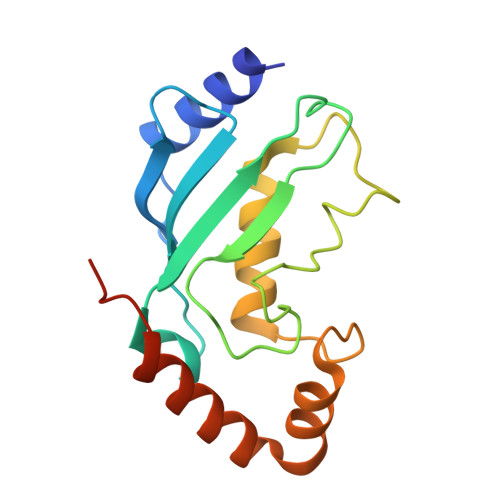 | |
UniProt | |||||
Find proteins for A5PJC4 (Bos taurus) Explore A5PJC4 Go to UniProtKB: A5PJC4 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A5PJC4 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ubiquitin-like protein ISG15 | 154 | Bos taurus | Mutation(s): 0 Gene Names: ISG15, G1P2, ISG17, UCRP | 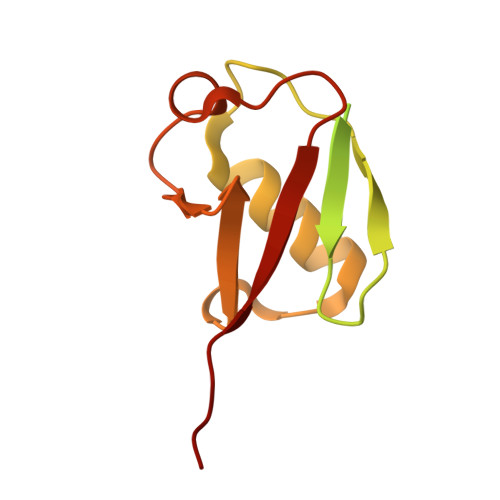 | |
UniProt | |||||
Find proteins for O02741 (Bos taurus) Explore O02741 Go to UniProtKB: O02741 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O02741 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| AMP (Subject of Investigation/LOI) Query on AMP | D [auth C] | ADENOSINE MONOPHOSPHATE C10 H14 N5 O7 P UDMBCSSLTHHNCD-KQYNXXCUSA-N |  | ||
| Funding Organization | Location | Grant Number |
|---|---|---|
| Academia Sinica (Taiwan) | Taiwan | -- |