Structural and functional insights into the T-even type bacteriophage topoisomerase II
Xin, Y.H., Chen, Y.T.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| DNA topoisomerase large subunit,DNA topoisomerase small subunit | A, C [auth B] | 682 | Tequatrovirus T4 | Mutation(s): 0 Gene Names: 39, 60 EC: 5.6.2.2 | 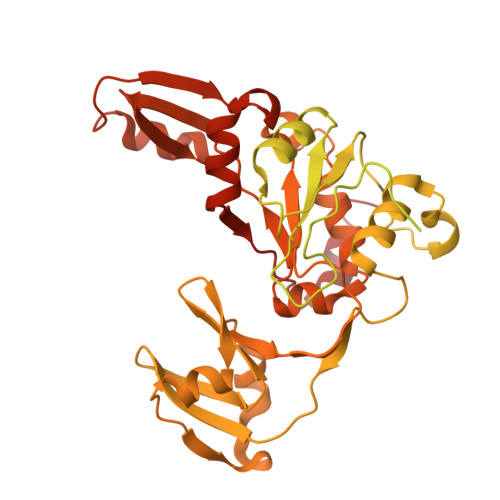 |
UniProt | |||||
Find proteins for P09176 (Enterobacteria phage T4) Explore P09176 Go to UniProtKB: P09176 | |||||
Find proteins for P23992 (Enterobacteria phage T4) Explore P23992 Go to UniProtKB: P23992 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Groups | P23992P09176 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| DNA topoisomerase medium subunit | B [auth C], D | 452 | Tequatrovirus T4 | Mutation(s): 0 Gene Names: 52 EC: 5.6.2.2 | 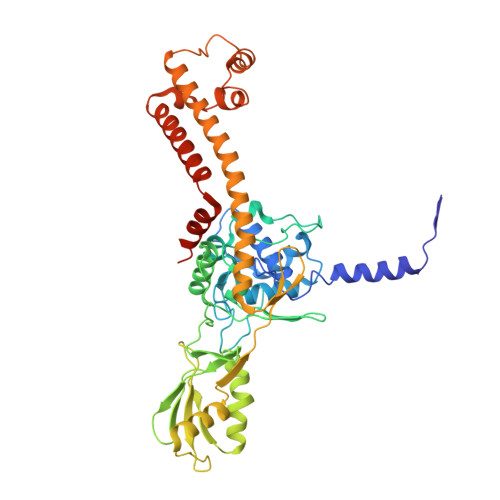 |
UniProt | |||||
Find proteins for P07065 (Enterobacteria phage T4) Explore P07065 Go to UniProtKB: P07065 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P07065 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| DNA (25-MER) | 25 | DNA molecule | 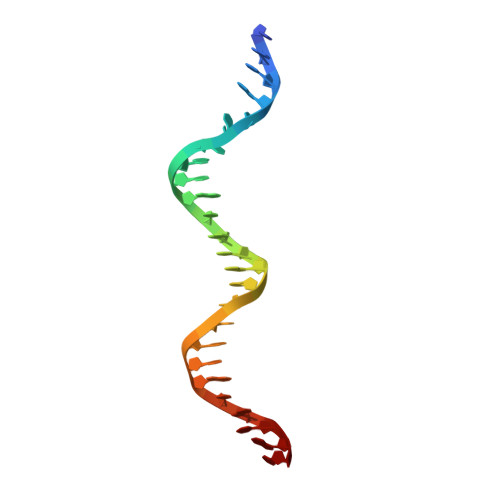 | ||
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| DNA (25-MER) | 25 | DNA molecule | 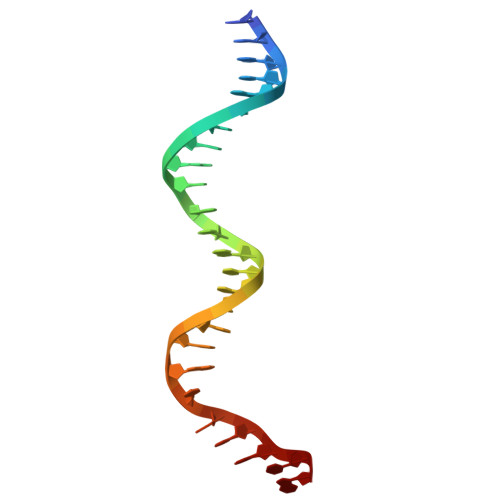 | ||
Sequence AnnotationsExpand | |||||
| |||||
| Funding Organization | Location | Grant Number |
|---|---|---|
| Chinese Academy of Sciences | China | -- |