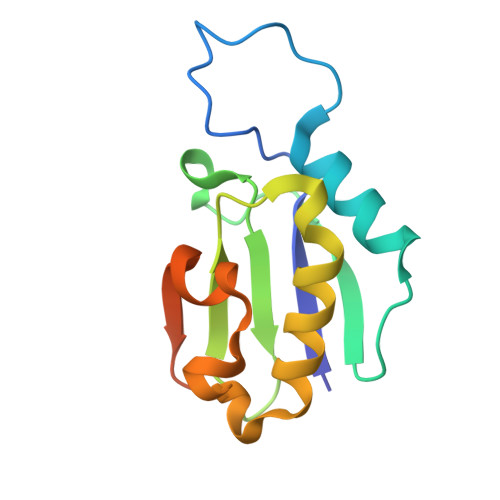
Crystal Structure of a Thioredoxin-like Ferredoxin Encoded Within a Cobalamin Biosynthetic Operon of Rhodobacter capsulatus.
Shen, Y.H., Cheng, W.L., Wang, X., Dai, H.E., Wang, M., Liu, L.(2025) Protein J 44: 192-200
- PubMed: 39924633
- DOI: https://doi.org/10.1007/s10930-025-10254-z
- Primary Citation of Related Structures:
9JN8 - PubMed Abstract:
Thioredoxin-like ferredoxin is a small homodimeric protein containing a [2Fe-2S] cluster in each monomer. It is only found in bacteria but its physiological function remains largely unknown. The cobalamin biosynthetic operon in the genome of the purple phototroph Rhodobacter capsulatus encodes a putative ferredoxin dubbed as CfrX. To characterize this protein, we cloned, expressed, purified, and crystalized the recombinant CfrX in the iron-sulfur cluster-bound state, and solved the structure at 2.1-Å resolution. Adopting a typical thioredoxin-like ferredoxin fold, a CfrX monomer binds one [2Fe-2S] cluster through four Cys residues located on two protruding loops. Unexpectedly, CfrX dimerizes in a previously unreported manner. With the structural information, we ascertained CfrX as a thioredoxin-like ferredoxin. While the precise function of CfrX in cobalamin biosynthesis is elusive, a link between CfrX and aerobic cobaltochelatase should exist due to the gene clustering pattern. We also discussed the possible relationship among CfrX, CobW, and CobNST with respect to the [2Fe-2S] cluster.
- School of Life Sciences, Anhui University, 111 Jiulong Road, Hefei, Anhui, 230601, China.
Organizational Affiliation: