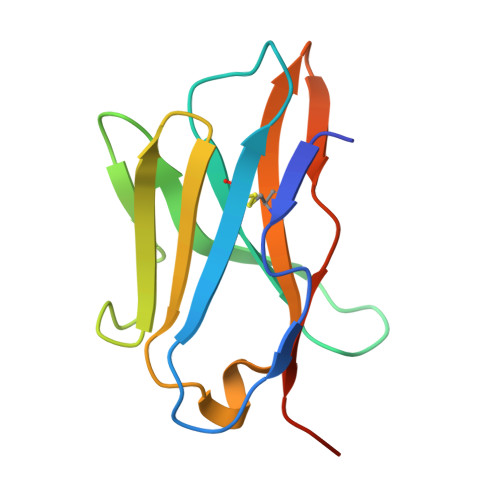
Insights into the structural features of a feline CD8 alpha alpha homodimer.
Liang, R.Y., Cao, Y.D., Gao, Y.Y., Li, H.Y., Sang, C.J., Xiu, Y.H., Li, D., Liu, D.Z., Gao, F.S., Li, Z.B.(2025) Dev Comp Immunol 169: 105405-105405
- PubMed: 40553752
- DOI: https://doi.org/10.1016/j.dci.2025.105405
- Primary Citation of Related Structures:
9JL0 - PubMed Abstract:
Cat ownership enjoys widespread popularity across the globe, with over 50% of households in certain countries owning feline companions. Besides providing outstanding emotional support for human, cats occupy a distinctive niche in scientific research, with their unique biological attributes and disease susceptibilities establishing them as a preferred model species across multiple disciplines. However, structural features and biological role of feline immune-related molecules remain largely unknown. In the current study, we crystallize feline CD8αα (fCD8αα) ectodomain and analyze its structural features. The fCD8αα ectodomain acts up to canonical fold of CD8αα homodimer immunoglobulin variable (Ig-V) and has a common feature of CD8α also is symbolic α-helix located on E-F loop. In line with the known CD8αα, the homodimer formation of fCD8αα relies upon the conserved hydrophobic core and conserved and species-specific residues provide additional hydrogen bonds. Compared with the other known CD8α monomers, the structural differences of fCD8α monomer focus on the loop regions linking different strands, which also show low sequence similarity in sequence alignment results. Further, we generate an fCD8αα/FLA-E*01801 model with high pLDDT and ipTM scores using AlphaFold3. Regardless of the spatial conformations of conserved or similar residues and possible interactions, the binding manner between fCD8αα and FLA-E*01801 complex closely resembles that of the known hCD8αα/HLA-A2 complex. Overall, our results provide structural and immunological knowledge for studying CD8αα function in the feline model and reveal the potential immunomodulatory roles of feline CD8 and its binding partners, deepening our understanding of the structural and functional mechanisms underlying feline immune responses.
- Key Laboratory of Animal Biosafety Risk Prevention and Control (North) and Key Laboratory of Veterinary Biological Products and Chemical Drugs of Ministry of Agriculture and Rural Affairs (MARA), Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing, China.
Organizational Affiliation: