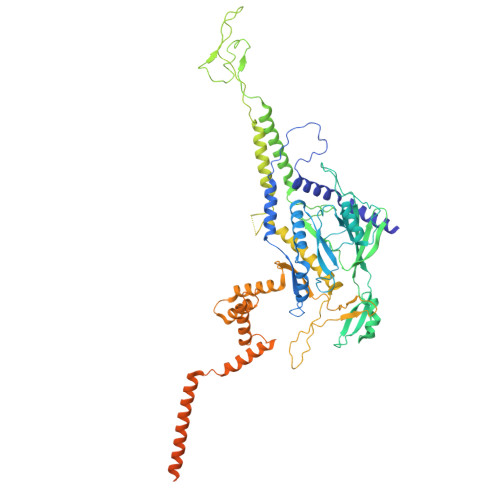
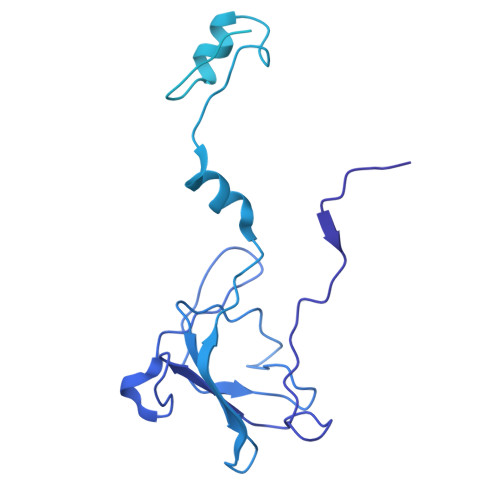
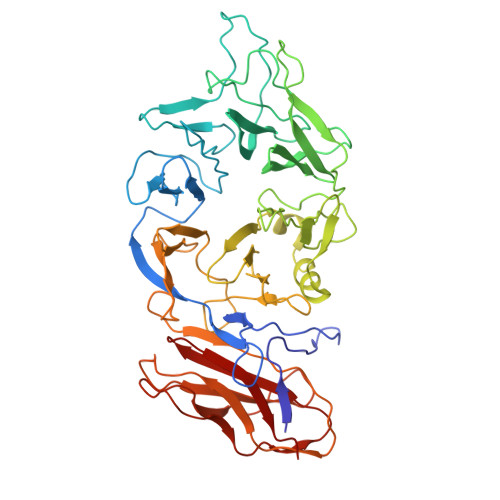
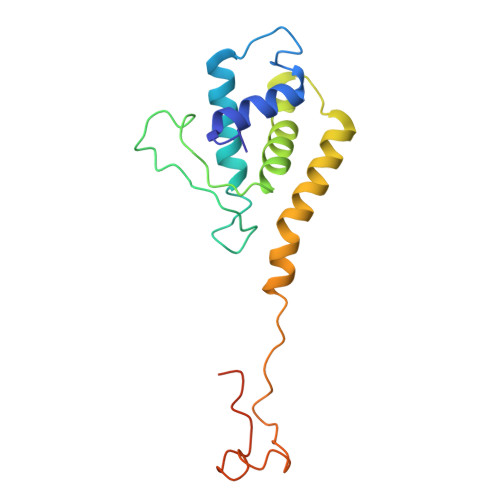
Structure of the scaffolding protein and portal within the bacteriophage P22 procapsid provides insights into the self-assembly process.
Xiao, H., Chen, W., Pang, H., Zheng, J., Wang, L., Feng, H., Song, J., Cheng, L., Liu, H.(2025) PLoS Biol 23: e3003104-e3003104
- PubMed: 40245015
- DOI: https://doi.org/10.1371/journal.pbio.3003104
- Primary Citation of Related Structures:
9JG6, 9JGA, 9KYV, 9KYW, 9KYX, 9KYY - PubMed Abstract:
In the assembly pathway of tailed double-stranded DNA (dsDNA) bacteriophages and herpesviruses, a procapsid with a dodecameric portal for DNA delivery at a unique vertex is initially formed. Appropriate procapsid assembly requires the transient presence of multiple copies of a scaffolding protein (SP), which is absent in the mature virion. However, how the SP contributes to dodecameric portal formation, facilitates portal and coat protein incorporation, and is subsequently released remains unclear because of a lack of structural information. Here, we present the structure of the SP-portal complex within the procapsid of bacteriophage P22 at 3-9 Å resolutions. The AlphaFold2-predicted SP model fits well with the density map of the complex. The SP forms trimers and tetramers that interact to yield a dome-like complex on the portal. Two SP domains mediate multimerization. Each trimer interacts with two neighboring portal subunits. The SP has a loop-hook-like structure that aids in coat protein recruitment during viral assembly. The loops of those SP subunits on the portal are positioned in clefts between adjacent portal subunits. Conformational changes in the portal during phage maturation may trigger the disassembly and release of the SP complex. Our findings provide insights into SP-assisted procapsid assembly in bacteriophage P22 and suggest that this strategy is also implemented by other dsDNA viruses, including herpesviruses.
- Institute of Interdisciplinary Studies, Key Laboratory for Matter Microstructure and Function of Hunan Province, Key Laboratory of Low-dimensional Quantum Structures and Quantum Control, School of Physics and Electronics, Hunan Normal University, Changsha, China.
Organizational Affiliation: