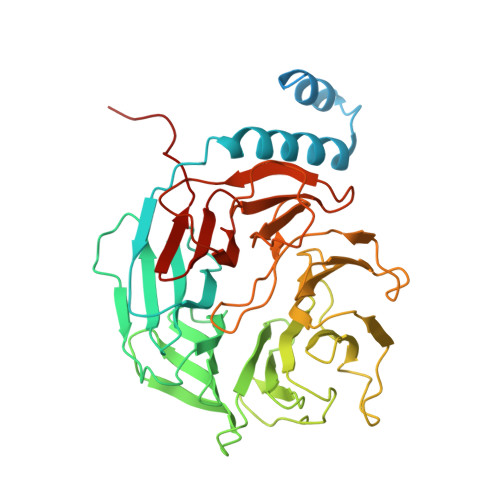
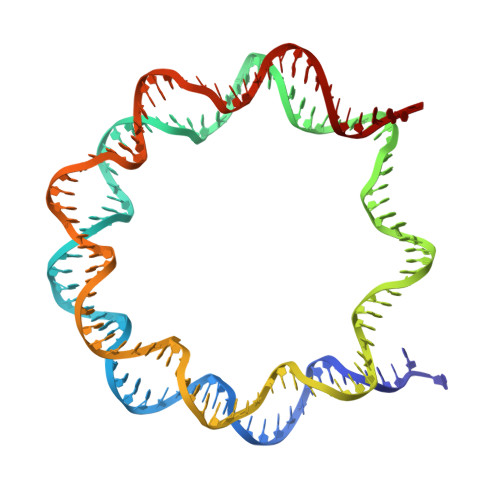
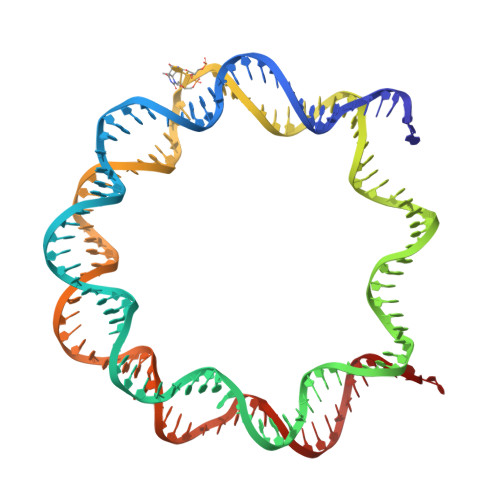
Structural basis of cyclobutane pyrimidine dimer recognition by UV-DDB in the nucleosome.
Matsumoto, S., Takizawa, Y., Ogasawara, M., Hashimoto, K., Negishi, L., Xu, W., Tachibana, H., Yamamoto, J., Iwai, S., Sugasawa, K., Kurumizaka, H.(2025) Nat Commun 16: 9709-9709
- PubMed: 41219227
- DOI: https://doi.org/10.1038/s41467-025-65486-5
- Primary Citation of Related Structures:
9J8W - PubMed Abstract:
In mammalian global genomic nucleotide excision repair, UV-DDB plays a central role in recognizing DNA lesions, such as 6-4 photoproducts and cyclobutane pyrimidine dimers, within chromatin. In the present study, we perform cryo-electron microscopy analyses coupled with chromatin-immunoprecipitation to reveal that the cellular UV-DDB binds to UV-damaged DNA lesions in a chromatin unit, the nucleosome, at a position approximately 20 base-pairs from the nucleosomal dyad in human cells. An alternative analysis of the in vitro reconstituted UV-DDB-cyclobutane pyrimidine dimer nucleosome structure demonstrates that the DDB2 subunit of UV-DDB specifically recognizes the cyclobutane pyrimidine dimer lesion at this position on the nucleosome. We also determine the structures of UV-DDB bound to DNA lesions at other positions in purified cellular human nucleosomes. These cellular and reconstituted UV-DDB-nucleosome complex structures provide important evidence for understanding the mechanism by which UV lesions in chromatin are recognized and repaired in mammalian cells.
- Laboratory of Chromatin Structure and Function, Institute for Quantitative Biosciences, The University of Tokyo, Bunkyo-ku, Tokyo, Japan.
Organizational Affiliation: