Transport and inhibition mechanisms of URAT1
Zhao, Y., Yu, Z.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Solute carrier family 22 member 12 | 553 | Rattus norvegicus | Mutation(s): 0 Gene Names: Slc22a12, Urat1 | 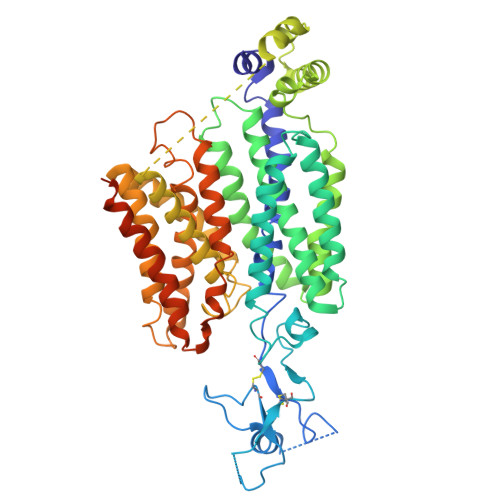 | |
UniProt | |||||
Find proteins for Q3ZAV1 (Rattus norvegicus) Explore Q3ZAV1 Go to UniProtKB: Q3ZAV1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q3ZAV1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| A1AIL (Subject of Investigation/LOI) Query on A1AIL | B [auth A] | lesinurad C17 H14 Br N3 O2 S FGQFOYHRJSUHMR-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| RECONSTRUCTION | cryoSPARC |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | -- |