High Resolution Crystal Structure of Glyceraldehyde-3-Phosphate Dehydrogenase from Saccharomyces cerevisiae Complexed with a Maleate Derivative
Cao, H., Zhang, X., Ren, Y., Wan, J.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Glyceraldehyde-3-phosphate dehydrogenase 3 | 340 | Saccharomyces cerevisiae S288C | Mutation(s): 0 Gene Names: TDH3, GPD3, YGR192C, G7576 EC: 1.2.1.12 | 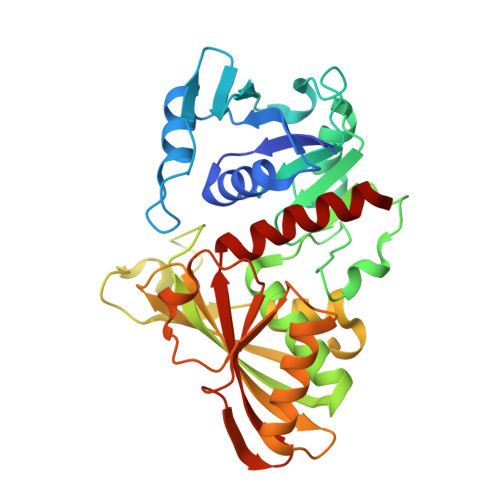 | |
UniProt | |||||
Find proteins for P00359 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore P00359 Go to UniProtKB: P00359 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00359 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| A1D98 (Subject of Investigation/LOI) Query on A1D98 | B [auth A] | methyl 4-[(4-ethynylphenyl)amino]-4-oxidanylidene-butanoate C13 H13 N O3 HMVIXCAGQZCUNY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 116.569 | α = 90 |
| b = 116.569 | β = 90 |
| c = 115.635 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| DIALS | data scaling |
| XDS | data reduction |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | 22177036 |
| National Natural Science Foundation of China (NSFC) | China | 22377030 |
| National Natural Science Foundation of China (NSFC) | China | 22373039 |