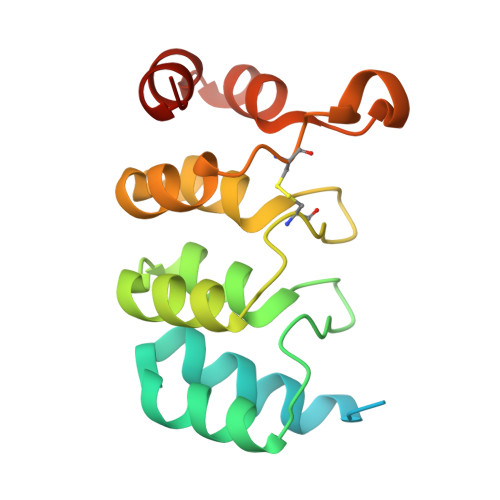
Structural analysis of a bacterial ankyrin-like protein secreted by Acinetobacter baumannii.
Sung, J.H., Lee, S.Y., Lee, C.S., Lee, J.H., Park, H.H.(2024) Biochem Biophys Res Commun 733: 150573-150573
- PubMed: 39208644
- DOI: https://doi.org/10.1016/j.bbrc.2024.150573
- Primary Citation of Related Structures:
9IQ8 - PubMed Abstract:
In bacteria, the ankyrin-like protein AnkB helps overcome stress by regulating catalase activity when expressed under stressful conditions. As the structural properties of AnkB are largely unexplored, our understanding of various AnkB-mediated functions in bacteria remains limited. In the present study, we describe the structure of AnkB from Acinetobacter baumannii, hereafter referred to as "AbAnkB," which has a unique tertiary configuration compared with that of other ankyrin domain-containing proteins. Structural analysis revealed that AbAnkB has a relatively long loop between AKR3 and AKR4 and an oppositely positioned α 8 helix. Based on amino acid conservation and protein surface analyses, we identified a hydrophobic patch that might be critical for the function of AbAnkB. To the best of our knowledge, our study is the first to report the structure of a bacterial AnkB protein; our findings will markedly enhance our understanding of its functions in bacteria.
- College of Pharmacy, Chung-Ang University, Seoul, 06974, Republic of Korea; Department of Global Innovative Drugs, Graduate School of Chung-Ang University, Seoul, 06974, Republic of Korea.
Organizational Affiliation: